COMPASSIONATE USE PROTOCOL
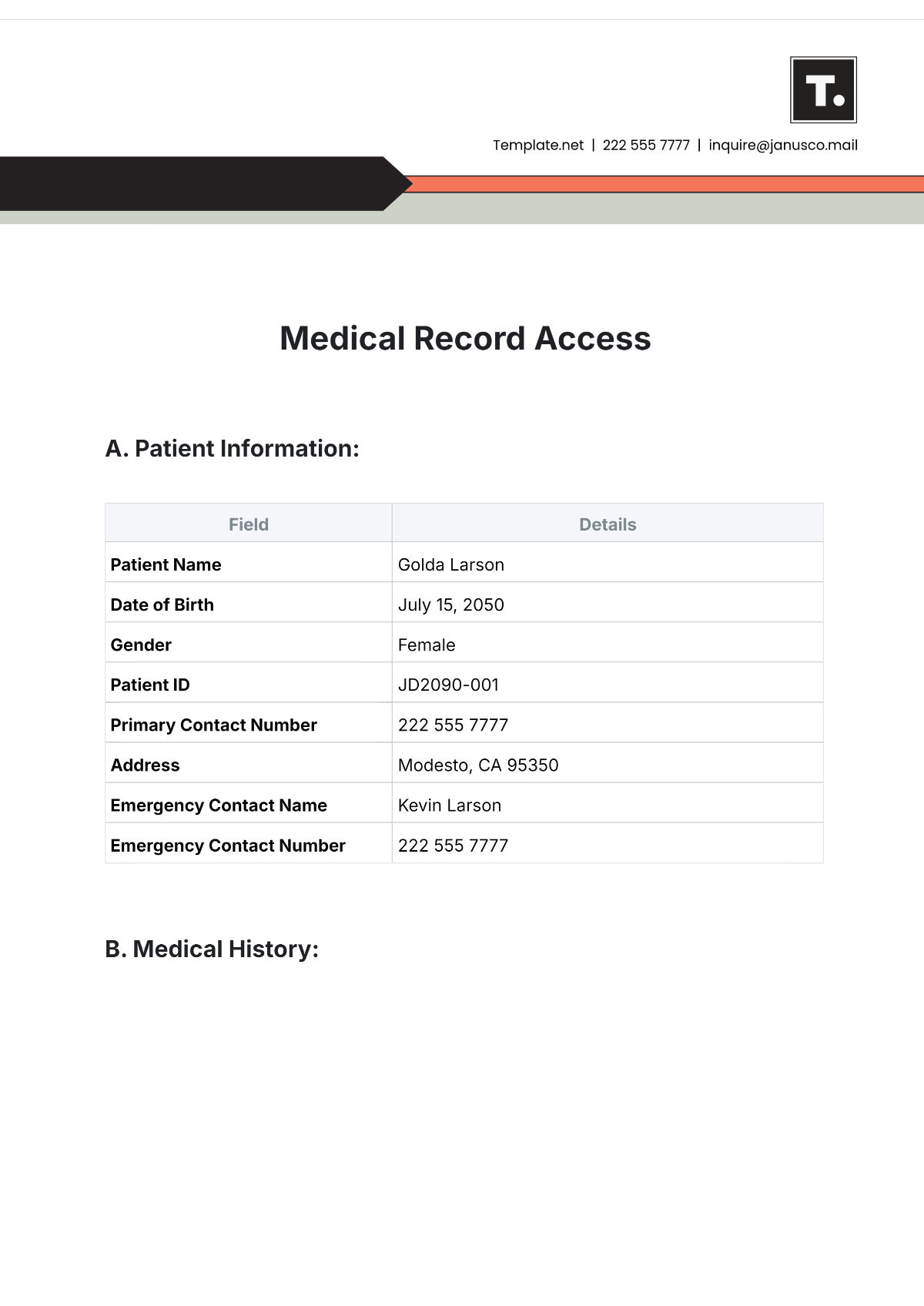
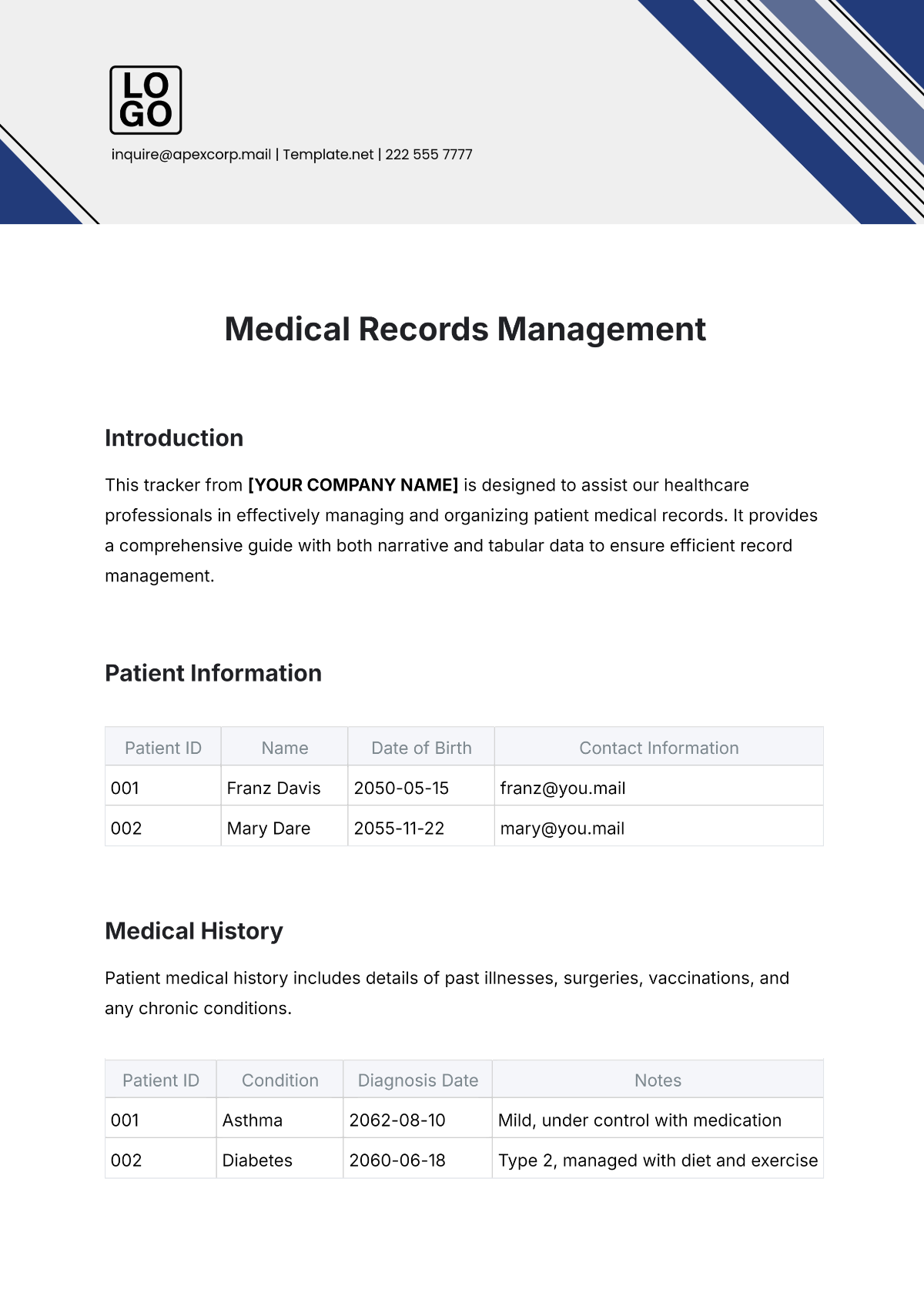
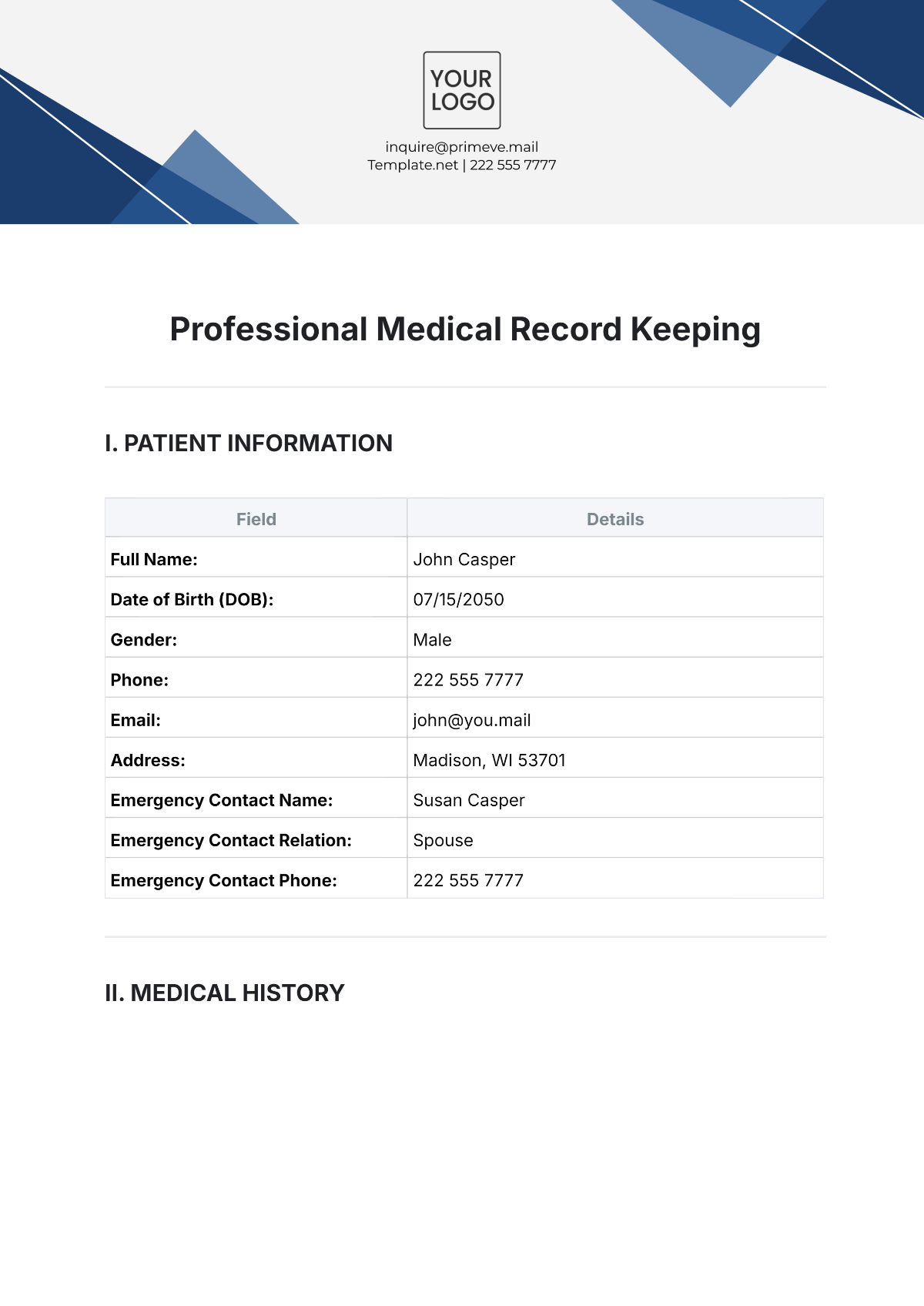
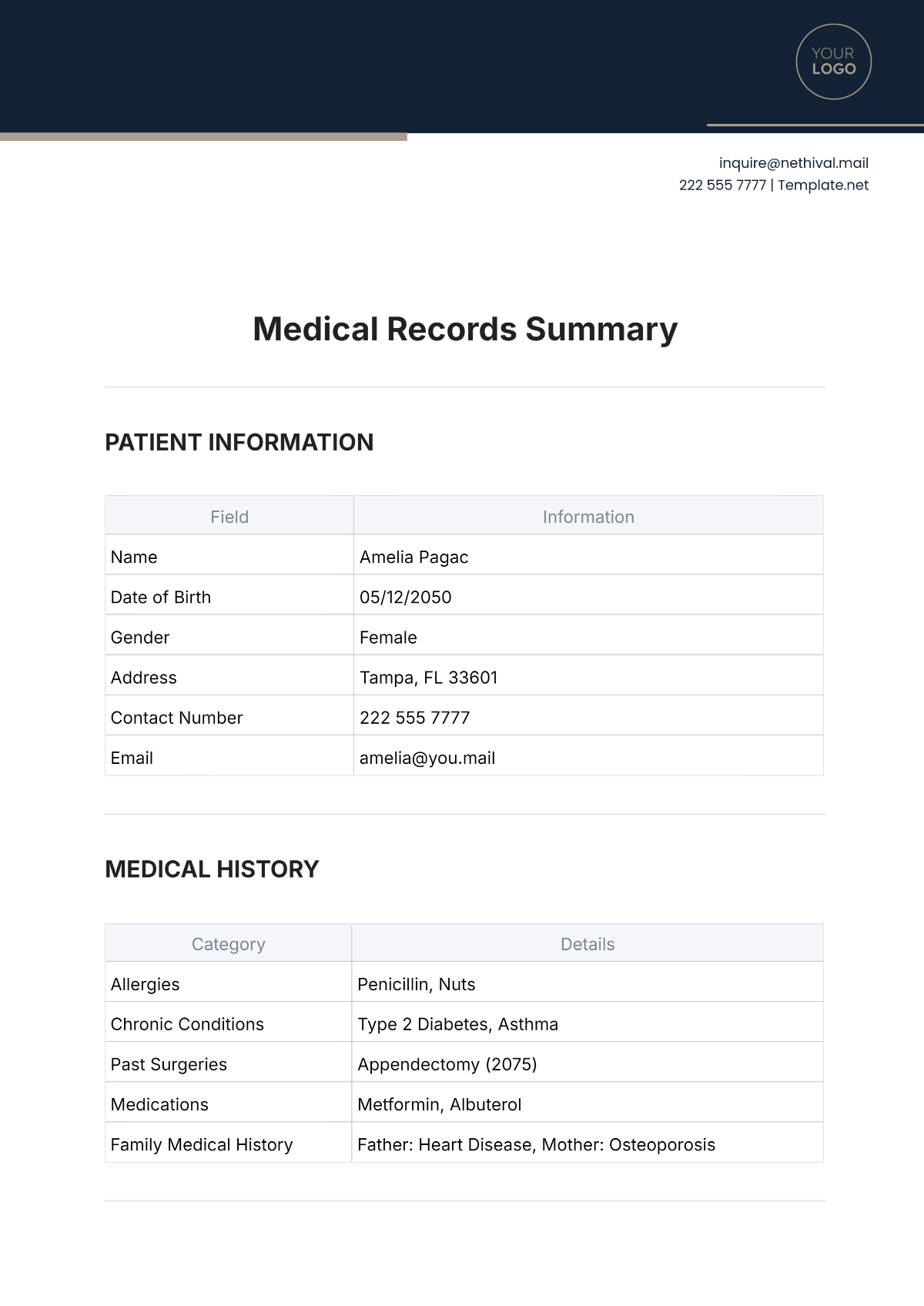
Name: | [Your Name] |
Company Name: | [Your Company Name] |
Department: | [YOUR DEPARTMENT] |
Date: | [DATE] |
I. Objectives
The main objective of this protocol is to establish a structured framework for providing investigational medical treatments to patients who have exhausted all other treatment options and are ineligible for existing clinical trials. Through this document, [Your Company Name] affirms its commitment to compassionate care by ensuring that all activities are conducted within the legal and ethical frameworks governing such interventions.
II. Protocol Overview
The Compassionate Use Protocol of [Your Company Name] is dedicated to providing experimental treatments that have not yet received full regulatory approval to patients with limited treatment options. This is done following a thorough assessment of risks, potential benefits, and ethical considerations.
Our Compassionate Use Program adheres to stringent protocols to ensure that the experimental treatments administered are in the best interests of the patients involved. This includes:
Risk Assessment: Prioritizing patient safety by assessing and mitigating potential risks associated with the experimental treatments.
Benefit Evaluation: Carefully evaluating the potential benefits of the treatments for each patient, considering their unique medical circumstances.
Ethical Considerations: Ensuring compliance with ethical guidelines and principles in all aspects of treatment administration and patient care.
Procedural Guidelines: Outlining clear procedures for treatment administration, monitoring, and follow-up care to ensure consistency and quality of care.
Patient Selection Criteria: Defining criteria for selecting patients eligible for compassionate use treatments based on medical necessity and lack of viable alternatives.
Safety Protocols: Implementing rigorous safety protocols to monitor treatment effects, manage adverse events, and ensure patient well-being throughout the treatment process.
III. Procedure
Procedure Step | Description |
|---|---|
1. Patient Identification | Identify patients who meet the criteria for compassionate treatment, typically those with a serious condition lacking alternative effective therapies. |
2. Safety Measures | Implement rigorous safety measures to minimize risks associated with experimental treatments and prioritize patient safety and well-being. |
3. Monitoring | Closely monitor treatment administration, efficacy, and adverse effects under the guidance of experienced healthcare professionals. |
4. Legal and Ethical Guidelines | Adhere strictly to legal and ethical guidelines throughout the treatment process, including obtaining informed consent and ensuring patient confidentiality. |
5. Transparency | Maintain full transparency in communication with patients and their families regarding treatment, risks, benefits, and available alternatives. |
IV. Data Collection
The data collection process is crucial within our compassionate use protocol. We employ structured methodologies to ensure accurate capture of various aspects:
Effectiveness: We measure the effectiveness of investigational treatments.
Adverse Reactions: We document any adverse reactions or side effects observed.
Patient Health: We track the overall health status and disease progression of patients.
All data collected is strictly confidential and serves multiple purposes:
Enhancing Understanding: Data aids in understanding treatment efficacy and safety.
Future Development: Insights gained contribute to the development of future remedies.
Informed Consent: Patients and their families are informed about data collection methods, purpose, and significance. Their informed consent is a priority throughout the process.
V. Safety Considerations
[Your Company Name]'s Compassionate Use Protocol prioritizes patient safety through comprehensive safety measures:
Dedicated Safety Team: We have a dedicated safety team responsible for assessing potential risks and ensuring patient well-being throughout the compassionate use treatment process.
Informed Patients and Families: Patients and their families are well-informed about the potential risks and benefits of the treatment option.
Emergency Handling: The protocol includes mechanisms for handling emergency scenarios promptly and effectively.
Adverse Event Management: We have procedures in place for dealing with serious adverse events that may occur during treatment.
Reporting and Compliance: Undesirable outcomes are reported to the relevant authorities in compliance with regulatory standards.
VI. Expected Results
The expected results from this protocol encompass multiple aspects:
Patient Relief: Our primary aim is to provide relief to patients by offering them a new potential treatment pathway. While we cannot guarantee efficacy, we earnestly hope that investigational treatments will contribute to improving patients' health conditions.
Insights into Treatment Effectiveness and Safety: Data obtained from these treatments will provide valuable insights into the effectiveness and safety of novel interventions. This knowledge can guide future treatment approaches and enhance patient care.
Expedited Development and Approval: The insights gained may expedite the further development and approval of these treatments, potentially benefiting a larger patient population in the future. This could lead to more accessible and effective treatment options for patients with unmet medical needs.
VII. Conclusion
The Compassionate Use Program of [Your Company Name] is dedicated to offering patients a chance at life through unapproved yet potentially lifesaving treatments. Our commitment to patient safety, ethical compliance, transparency, and advancing medical science drives our efforts.