IRB Protocol
Name | [YOUR NAME] |
|---|---|
Company | [YOUR COMPANY NAME] |
Department | [YOUR DEPARTMENT] |
Date | [DATE] |
1. Introduction
This protocol outlines the procedures and methods for conducting the research study titled "The Effects of Exercise on Blood Pressure Management in Hypertensive Patients." The study aims to investigate the impact of a structured exercise program on blood pressure control among individuals diagnosed with hypertension.
2. Study Objectives
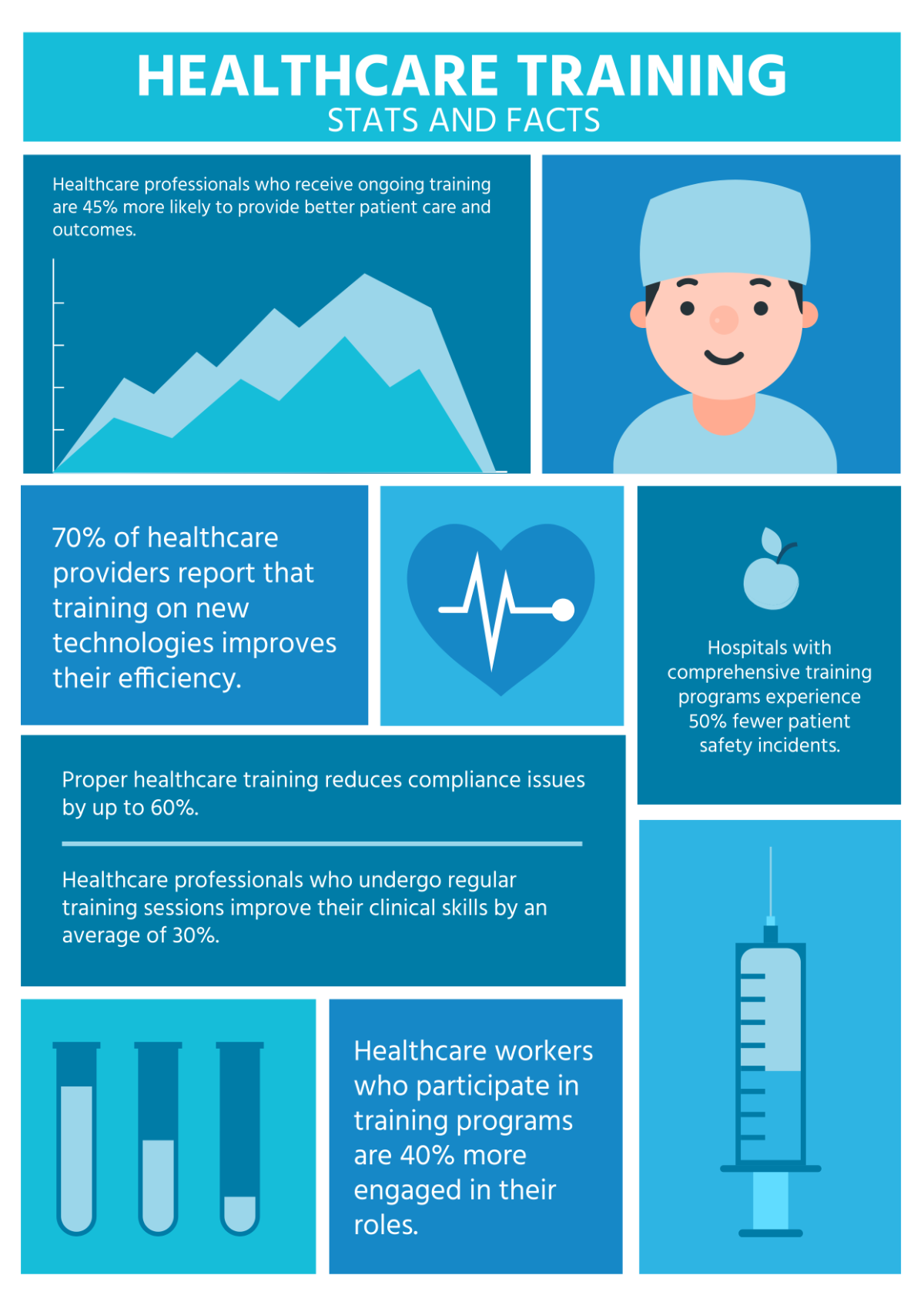
The primary objective of this study is to assess the effectiveness of a 12-week exercise intervention in reducing systolic and diastolic blood pressure levels in hypertensive patients. Secondary objectives include evaluating changes in participants' cardiovascular fitness levels and adherence to the exercise program.
3. Methodology
The study will employ a randomized controlled trial design. Hypertensive patients aged 40-65 years will be randomly assigned to either the exercise intervention group or the control group. Participants in the intervention group will undergo a supervised exercise program consisting of aerobic and resistance training sessions three times per week. Blood pressure measurements will be taken before and after the intervention period using standardized procedures.
4. Participant Recruitment Process
Criteria | Details |
|---|---|
Inclusion Criteria | - Diagnosed with hypertension |
- Aged between 40-65 years | |
- Medically cleared for participation in an exercise program | |
Exclusion Criteria | - Unstable cardiovascular conditions |
- History of musculoskeletal injuries |
Participants will be recruited from the cardiology clinic at [Name of Hospital] based on the following inclusion criteria. Recruitment methods will include physician referrals and poster advertisements placed in the clinic waiting area. Potential participants will receive an information sheet outlining the study aims, procedures, and potential risks and benefits.
5. Data Collection Procedures
Data Collection | Details |
|---|---|
Baseline Assessments | - Blood pressure measurements |
- Demographic information | |
- Baseline fitness assessments | |
- Medical history | |
Measurement Tools | - Automated blood pressure monitor |
- 6-minute walk test | |
- Sit-to-stand test | |
Location | Cardiology Clinic |
Data will be collected through baseline assessments, including blood pressure measurements, demographic information, and baseline fitness assessments. Participants' blood pressure will be measured using an automated blood pressure monitor following standardized protocols. Fitness assessments will include the 6-minute walk test and the sit-to-stand test. Data collection will take place at the cardiology clinic.
6. Participant Safety and Confidentiality
Participant safety will be ensured by conducting pre-participation health screenings to identify any contraindications to exercise. The exercise program will be supervised by qualified fitness professionals, and participants will be closely monitored for any signs of distress during exercise sessions. Confidentiality will be maintained by assigning participants unique study identification numbers and storing all study-related data in password-protected electronic files.
7. Informed Consent
Informed consent will be obtained from all participants before they participate in the study. The informed consent process will include providing detailed information about the study aims, procedures, potential risks and benefits, and the voluntary nature of participation. Participants will be given ample opportunity to ask questions, and written consent will be obtained from those willing to participate.
8. Conclusion
This protocol outlines the key aspects of the research study, including its objectives, methodology, participant recruitment process, data collection procedures, measures for ensuring participant safety and confidentiality, and the informed consent process. The study aims to contribute valuable insights into the role of exercise in blood pressure management among hypertensive patients. Thank you for considering this protocol for review.