Free Science & Chemistry Datasheet
I. Introduction

Welcome to the [Your Company Name] Science & Chemistry Datasheet, your comprehensive guide to the safe handling, storage, and regulatory compliance of chemicals produced by [Your Company Name]. This datasheet aims to provide laboratory technicians with detailed information necessary for their work in ensuring safety and adherence to industry standards.
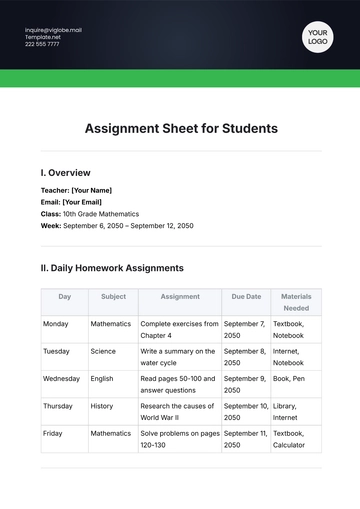
II. Chemical Information
A. Chemical Name and Formula
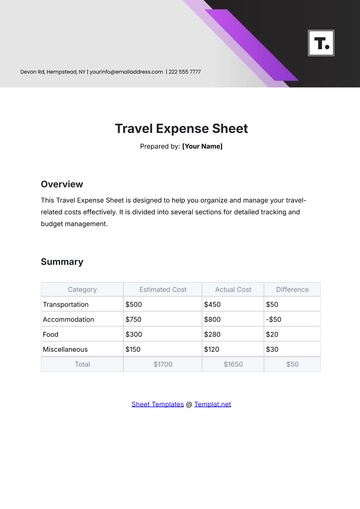
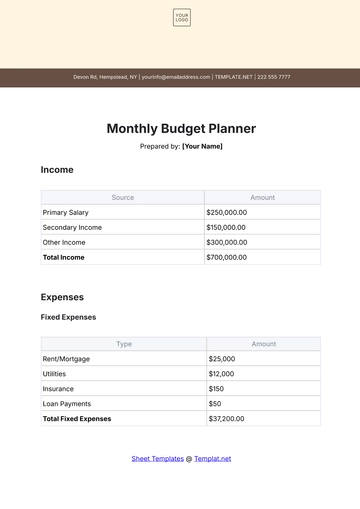
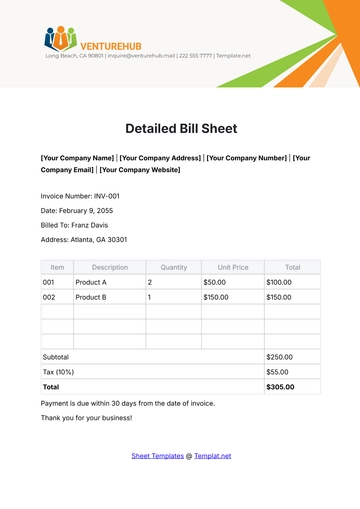
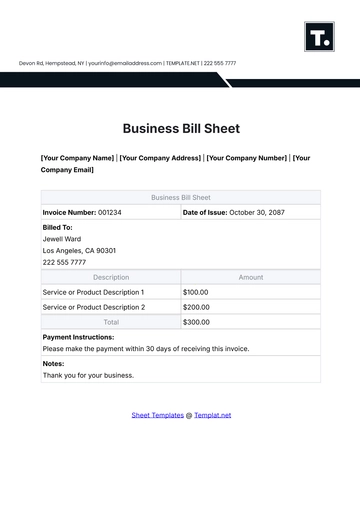
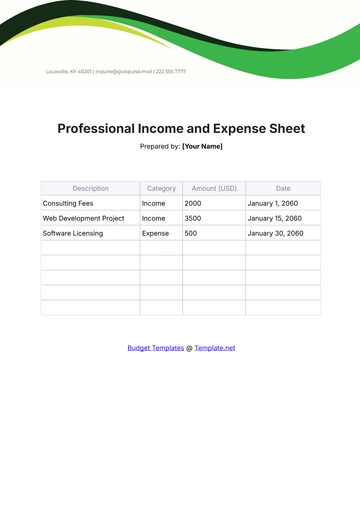
Aspect | Details |
|---|---|
Chemical Name | Sodium Chloride |
Chemical Formula | NaCl |
CAS Number | 7647-14-5 |
Structure | Sodium Chloride (NaCl) is a simple ionic compound composed of sodium ions (Na+) and chloride ions (Cl-), arranged in a cubic crystal lattice structure. |
B. Physical and Chemical Properties
Physical Properties:
Appearance: Sodium Chloride appears as a white crystalline solid.
Odor: It is odorless.
Melting Point: The melting point of Sodium Chloride is 801 degrees Celsius.
Boiling Point: It has a high boiling point of 1465 degrees Celsius.
Density: The density of Sodium Chloride is approximately 2.16 grams per cubic centimeter.
Solubility: It is highly soluble in water, forming a clear and colorless solution.
Chemical Properties:
Reactivity: Sodium Chloride is stable under normal conditions and does not undergo significant chemical reactions.
Compatibility: It is compatible with most common chemicals and materials used in laboratory settings.
III. Hazards and Safety Information

A. Hazards Identification
Toxicity: Sodium Chloride is considered non-toxic and poses no significant health risks under normal handling and use.
Flammability: It is non-flammable, posing no fire hazards.
Corrosivity: Sodium Chloride is non-corrosive and does not cause damage to metals or other materials.
Environmental Hazards: It does not present significant environmental hazards and is not harmful to aquatic life.
B. Handling Instructions
Always wear appropriate PPE (Personal Protective Equipment) such as gloves and goggles when handling Sodium Chloride.
Avoid inhalation and skin contact. In case of contact, rinse affected areas thoroughly with water.
Use Sodium Chloride in a well-ventilated area to minimize exposure.
C. Storage Recommendations
Store Sodium Chloride in a tightly closed container in a dry, well-ventilated area away from heat sources.
Keep it separated from incompatible materials and follow proper segregation guidelines.
D. Emergency Response
In case of ingestion, rinse your mouth and drink plenty of water. Seek medical attention if symptoms persist.
For skin contact, remove contaminated clothing and wash affected areas with soap and water.
If inhaled, move to fresh air and seek medical advice if respiratory irritation occurs.
For spills, contain the area, absorb the spill with suitable materials, and dispose of waste properly according to regulations.
IV. Regulatory Compliance
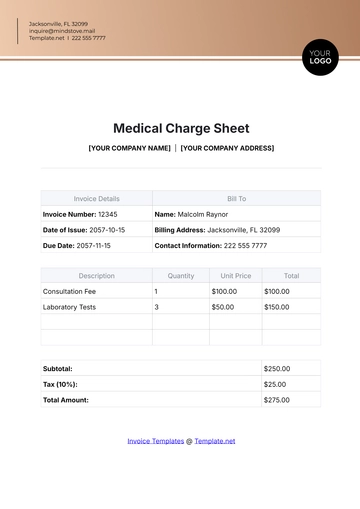
Compliance with Regulations: Sodium Chloride complies with OSHA (Occupational Safety and Health Administration) regulations for chemical handling and workplace safety.
Labeling Requirements: Ensure proper labeling of containers with the chemical name, hazards, handling instructions, and emergency contact information.
Safety Data Sheet (SDS): Refer to the Safety Data Sheet (SDS) for comprehensive information on hazards, handling, and emergency procedures specific to Sodium Chloride.
V. Additional Information

Uses: Sodium Chloride is commonly used in various industries such as food processing, pharmaceuticals, water treatment, and chemical manufacturing.
Storage Conditions: Store Sodium Chloride containers tightly closed in a cool, dry place away from direct sunlight and heat sources.
Transportation: Follow transportation regulations for hazardous materials when transporting Sodium Chloride to ensure safe handling and delivery.
Disposal: Dispose of Sodium Chloride waste in accordance with local regulations and environmental guidelines. Do not release into the environment.
VI. Contact Information
For further inquiries or assistance, please contact:
Manufacturer: [Your Company Name]
Address: [Your Company Address]
Phone Number: [Your Company Number]
Email: [Your Company Email]
Website: [Your Company Website]
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Document scientific data accurately with Template.net's Science & Chemistry Datasheet Template. This editable and customizable tool helps you record chemical properties, experiment results, and safety guidelines. Create detailed datasheets for research, education, and industry use. Editable in our AI Editor Tool, this template ensures precise and thorough scientific documentation, supporting effective communication and knowledge sharing in science and chemistry fields.
You may also like
- Attendance Sheet
- Work Sheet
- Sheet Cost
- Expense Sheet
- Tracker Sheet
- Student Sheet
- Tracking Sheet
- Blank Sheet
- Information Sheet
- Sales Sheet
- Record Sheet
- Price Sheet
- Plan Sheet
- Score Sheet
- Estimate Sheet
- Evaluation Sheet
- Checklist Sheet
- Bid Sheet
- Call Log Sheet
- Bill Sheet
- Assessment Sheet
- Task Sheet
- School Sheet
- Work From Home Sheet
- Summary Sheet
- Construction Sheet
- Cover Sheet
- Debt Spreadsheet
- Debt Sheet
- Client Information Sheet
- University Sheet
- Freelancer Sheet
- Bookkeeping Sheet
- Itinerary Spreadsheet
- Scorecard Sheet
- Run Sheet
- Monthly Timesheet
- Event Sheet
- Advertising Agency Sheet
- Missing Numbers Worksheet
- Training Sheet
- Production Sheet
- Mortgage Sheet
- Answer Sheet
- Excel Sheet