Bakery Ingredient Handling SOP Outline
I. Introduction
A. Purpose
(Describe the rationale and the goal of the SOP. Mention the importance of properly handling ingredients to maintain quality, safety, and compliance.)
B. Scope
(Detail the scope of the SOP. Explain who it applies to and which procedures are covered. Mention the various sections of the bakery operations that will follow this SOP.)
C. Responsibilities
(Specify the individuals and roles responsible for carrying out the SOP. Detail the responsibilities of bakers, supervisors, and quality control personnel.)
II. Receiving Ingredients
A. Inspection
(Provide detailed instructions on how to inspect incoming ingredients. Mention checking for expired dates, damage, and contamination.)
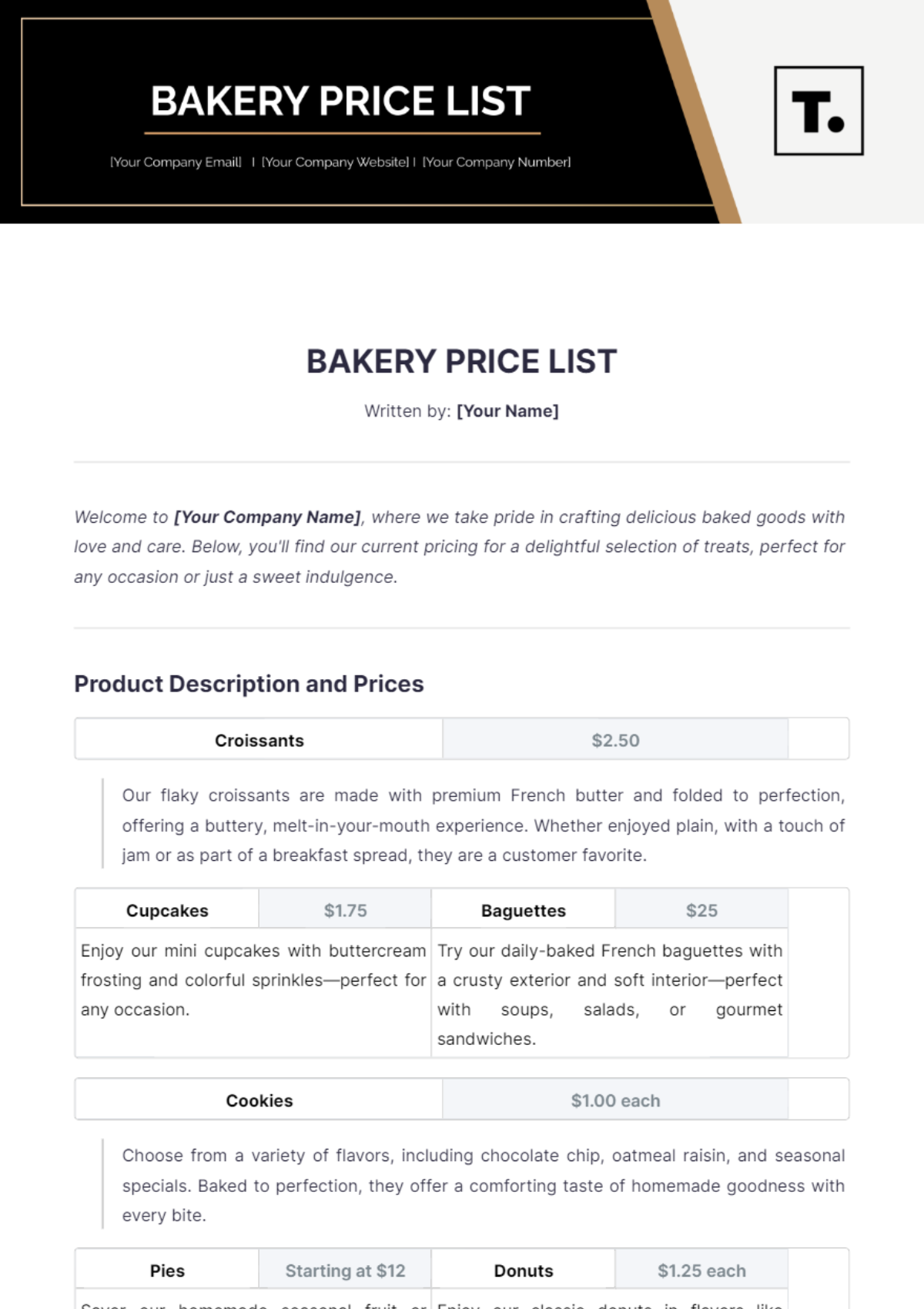
Ingredient | Supplier | Quantity | Date Received | Inspector |
|---|---|---|---|---|
[Flour] | [Supplies Co] | [100 kg] | [April 10, 2050] | [Jane Whitman] |
B. Documentation
(Explain the need for recording inventory and maintaining logs of received ingredients. Include maintaining records of supplier information and dates.)
III. Storing Ingredients
A. Temperature Control
(Outline the required storage temperatures for various ingredients. Mention refrigeration for perishables and dry storage for non-perishables.)
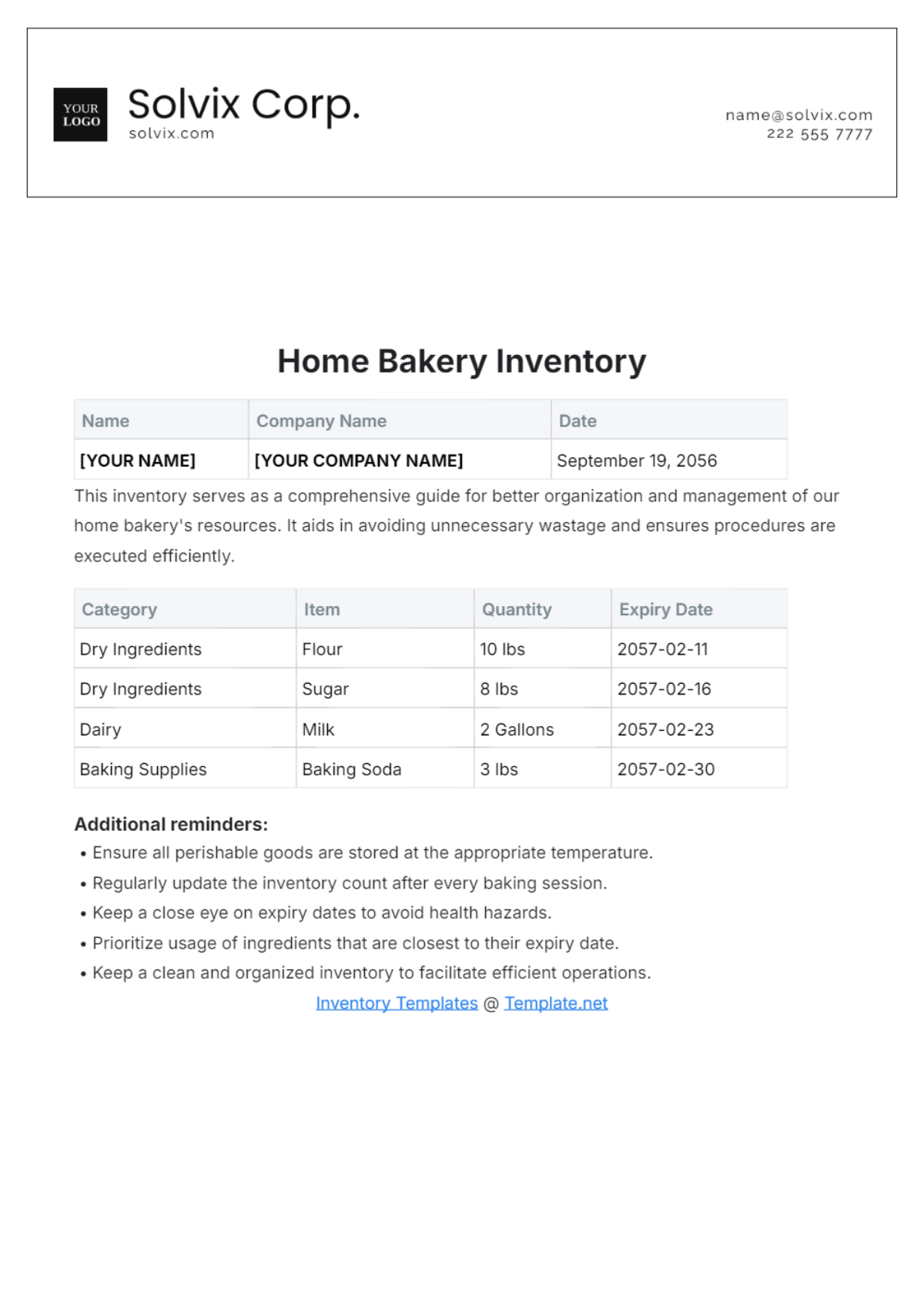
Ingredient | Storage Location | Storage Temperature | Expiry Date |
|---|---|---|---|
[Butter] | [Refrigerator] | [4°C] | [May 24, 2060] |
B. Stock Rotation
(Emphasize the importance of First-In-First-Out (FIFO) practices. Provide instructions on how to implement FIFO.)
IV. Handling and Weighing Ingredients
A. Cleanliness
(Specify the need for cleanliness and sanitation. Mention washing hands and using clean utensils before handling ingredients.)
B. Accuracy
(Guide on the importance of accurate measurements. Provide instructions on how to weigh ingredients correctly using calibrated scales.)
V. Mixing and Blending
A. Equipment Calibration
(Detail the steps for ensuring mixing and blending equipment is properly calibrated before use. Mention any specific checks to be performed.)
B. Mixing Instructions
(Provide detailed mixing instructions, including the sequence of adding ingredients and mixing times. Mention different mixing speeds if applicable.)
VI. Quality Control
A. Sampling
(Explain the procedure for sampling batches for quality control. Include the frequency and quantity of samples to be taken.)
B. Testing
(Provide details on the various tests that should be performed on samples. Mention specific characteristics to be tested, like moisture content and pH levels.)
VII. Packaging
A. Materials
(List the materials required for packaging baked goods. Mention the suitability of materials for different types of baked products.)
B. Sealing
(Describe the proper method for sealing packages to ensure freshness and prevent contamination. Include instructions for using sealing equipment if applicable.)
VIII. Labeling
A. Information
(Detail the information that needs to be included on labels. Mention items like ingredient list, batch number, expiry date, and nutritional information.)
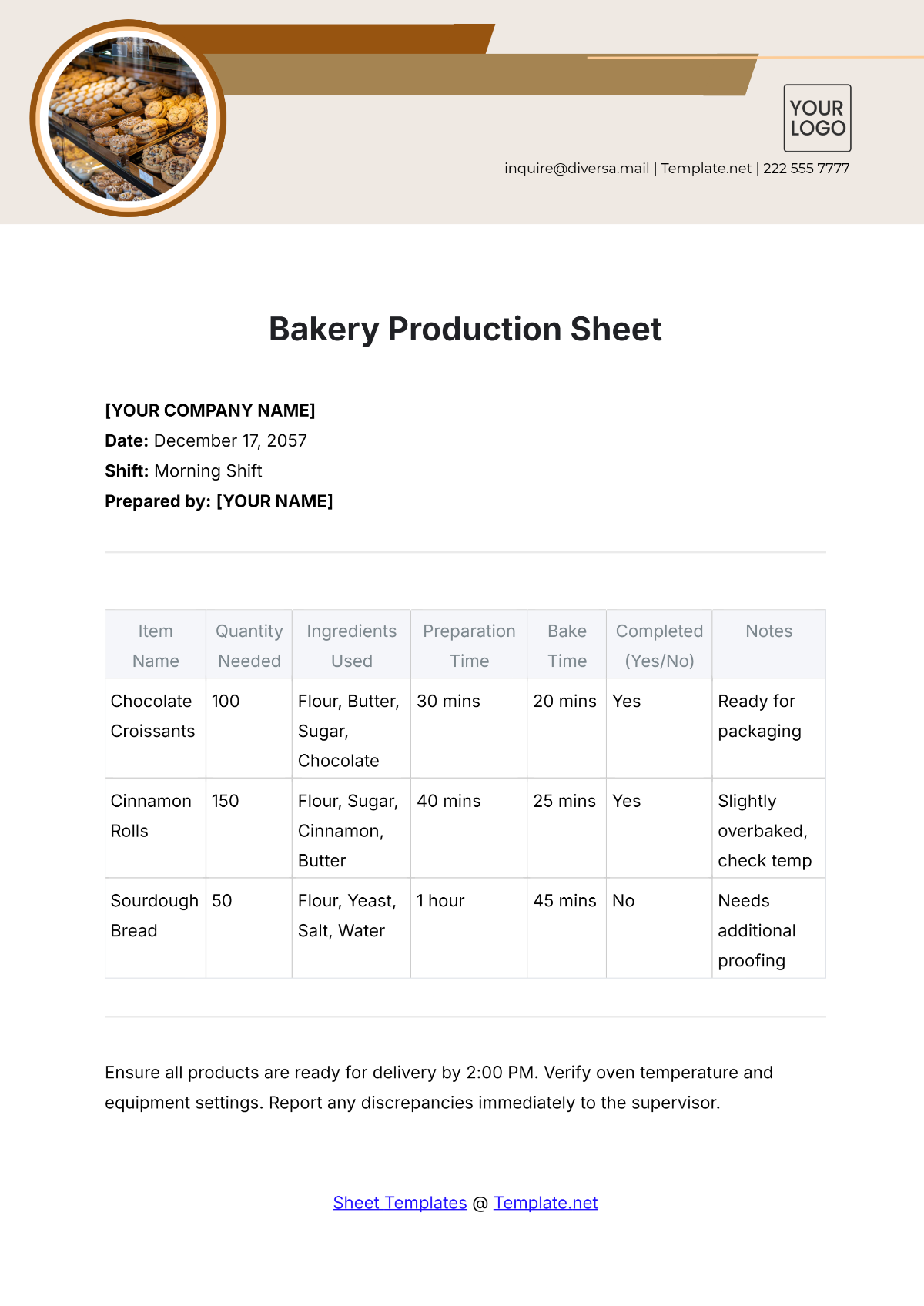
Product | Batch Number | Packaging Date | Expiry Date | Labeler |
|---|---|---|---|---|
[Chocolate Cake] | [CHC-001] | [August 10, 2055] | [May 18, 2057] | [Michael Green] |
B. Compliance
(Ensure that labeling meets regulatory requirements. Mention any specific laws or guidelines relevant to labeling.)
IX. Storage of Finished Products
A. Conditions
(Describe the optimal storage conditions for finished products. Mention temperature, humidity, and light exposure requirements.)
B. Inventory Management
(Provide instructions for tracking and managing inventory of finished products. Mention the use of inventory management software if applicable.)
X. Distribution
A. Preparation
(Detail the steps for preparing products for distribution. Include instructions for organizing orders and ensuring all packaging is secure.)
B. Transportation
(Provide guidelines for transporting baked goods. Mention the need for maintaining temperature and minimizing movement to prevent damage.)
XI. Record Keeping
A. Logs and Reports
(Explain the importance of maintaining logs and reports. Mention the types of records that should be kept and the duration for which they should be retained.)
Document | Responsible Person | Frequency | Retention Period |
|---|---|---|---|
[Quality Control Reports] | [Robert Johnson] | [Weekly] | [2 Years] |
B. Audits
(Provide instructions for conducting regular audits. Mention the frequency of audits and the aspects to be reviewed during audits.)
XII. Training
A. New Employee Orientation
(Detail the training that new employees must undergo. Include topics like ingredient handling, hygiene, and safety procedures.)
B. Continuing Education
(Provide guidelines for ongoing training. Mention the importance of refresher courses and staying updated on best practices and regulatory changes.)
XIII. Review and Revision
A. Regular Reviews
(Describe the schedule and process for regular reviews of the SOP. Include who is responsible for conducting reviews and what they should look for.)
B. Revision Process
(Detail the process for revising the SOP. Include how changes are documented, communicated, and implemented.)
C. Approval
(Outline the approval process for any revisions. Include the roles of management and quality control in approving changes.)