Designing Trials Research Design
Prepared By: [Your Name]
I. Introduction
Designing research trials is a pivotal aspect of scientific inquiry across various disciplines, including medicine, psychology, and social sciences. The trial design significantly affects the reliability, validity, and applicability of the results. This guide provides a structured approach to developing robust and effective trials, ensuring that research findings are both credible and impactful.
II. Basic Concepts in Trial Design
Hypothesis: A clear, testable statement predicting the trial's outcome, which guides the research objectives and methodology.
Variables: Elements of the study that are manipulated or measured. These include:
Independent Variables: Factors that are deliberately changed or controlled.
Dependent Variables: Responses to changes in independent variables.
Control Variables: Controlled variables ensure effects are due to independent variables.
Sample: A subset of the population selected to participate in the trial, which should represent the broader population to ensure generalizability.
Randomization: A process that assigns participants to different groups randomly to minimize bias and control for confounding variables.
Blinding: A technique used to prevent bias by concealing group assignments from participants, researchers, or both, ensuring that expectations do not influence the results.
III. Types of Research Designs
Experimental Design: This involves actively manipulating variables and using random assignment to establish cause-and-effect relationships. It is considered the gold standard for testing hypotheses due to its ability to control for confounding variables.
Quasi-Experimental Design: Similar to experimental design but lacks random assignment. This design is useful in real-world settings where randomization is impractical but makes establishing causality more challenging.
Observational Design: This design involves observing and measuring variables without manipulation. It is ideal for identifying correlations and understanding natural variations, but it cannot establish causation.
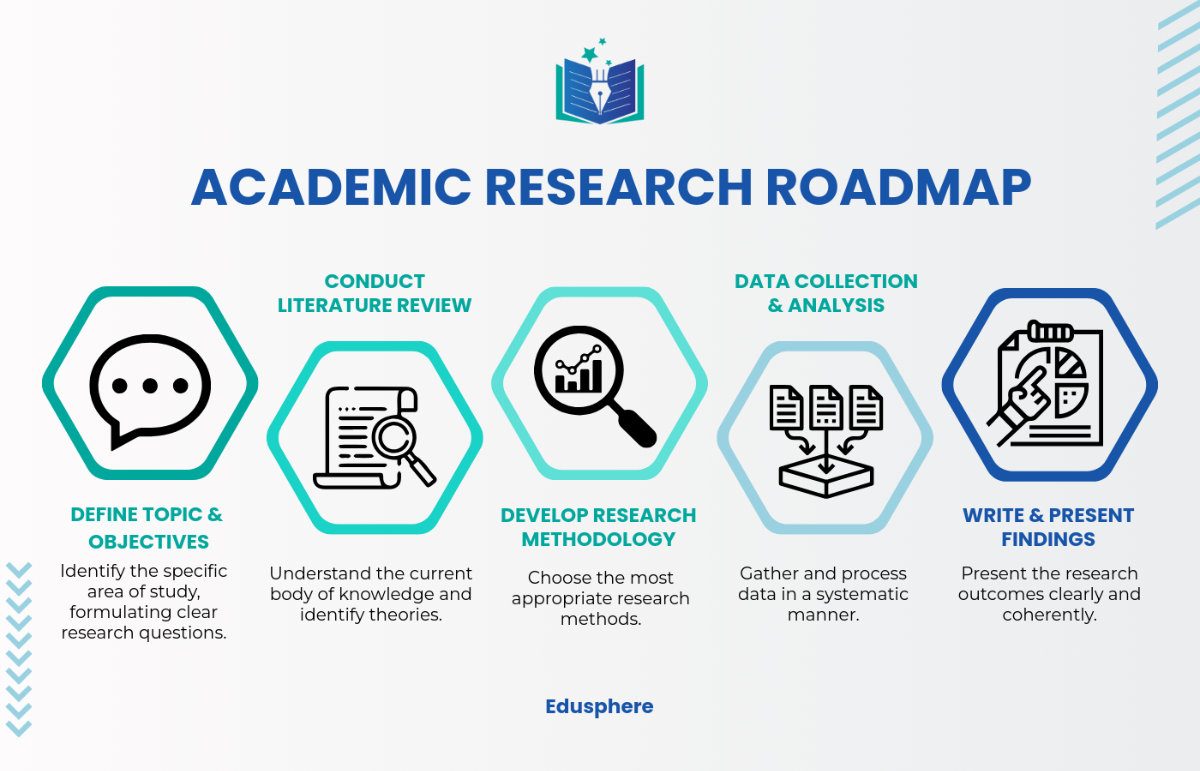
IV. Steps in Designing a Trial
Defining Objectives: Clearly articulate both primary and secondary objectives. These objectives will guide the formulation of the hypothesis and the overall scope of the research.
Literature Review: Perform a comprehensive review of existing literature to identify gaps in knowledge and build on previous research. This step refines the research question and hypothesis, ensuring that the trial addresses an unresolved issue.
Choosing the Methodology: Select the research design that best fits the objectives and nature of the study. Consider factors such as feasibility, ethical implications, and available resources.
Defining Variables: Identify and define:
Independent Variables: Factors you manipulate to see their effect.
Dependent Variables: Outcomes you measure to assess the impact of the independent variables.
Control Variables: Factors you keep constant to ensure the reliability of the results.
Sampling: Determine the sample size and sampling method. Common methods include:
Random Sampling: Every member of the population has an equal chance of being selected.
Stratified Sampling: The population is divided into subgroups, and samples are drawn from each subgroup to ensure representation.
Randomization and Blinding: Implement randomization to distribute confounding variables evenly across groups. Use blinding techniques to reduce bias, ensuring that neither participants nor researchers influence the outcome unintentionally.
Data Collection Methods: Choose appropriate methods for data collection, such as surveys, interviews, observations, or physiological measurements. Ensure these methods are reliable and valid, and pilot test them if necessary.
Ethical Considerations: Obtain approval from relevant ethics boards and ensure informed consent from participants. Address confidentiality, risk minimization, and the potential benefits of the research to ensure ethical integrity.
Data Analysis: Apply suitable statistical methods to analyze the data. Select techniques that align with the research design and objectives, such as regression analysis, t-tests, or ANOVA. Interpret the results in the context of the hypothesis and research questions.
Reporting Findings: Present results in a structured format, including:
Introduction: Background and research question.
Methodology: Detailed description of the design, methods, and procedures.
Results: Findings of the study, supported by appropriate statistics.
Discussion: Interpretation of results, implications, and limitations.
Conclusion: Summary of the findings and their significance.
V. Common Challenges and Solutions in Trial Design
Bias: Minimize bias through rigorous randomization and blinding techniques.
Sample Size: Use power analysis to determine an adequate sample size, balancing statistical power with practical constraints.
Ethical Concerns: Adhere to ethical guidelines, ensuring participant welfare and obtaining necessary approvals.
Data Quality: Use validated instruments and consistent procedures for data collection and entry to maintain high-quality data.
VI. Conclusion
Effective trial design involves careful planning and consideration of various factors, including objectives, methodology, and ethical issues. By following structured guidelines and best practices, researchers can enhance the reliability and validity of their findings, contributing valuable insights to their fields of study.
VII. References
Creswell, J. W. (2014). Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Sage Publications.
Polit, D. F., & Beck, C. T. (2017). Nursing Research: Generating and Assessing Evidence for Nursing Practice. Wolters Kluwer.
Rees, C. (2011). Introduction to Research for Midwives. Elsevier Health Sciences.