Medical Device Technical Specification
1. Introduction
1.1 Purpose
This document outlines the technical specifications for the Glucometer X1, hereinafter referred to as "the Device." The purpose of this specification is to provide a comprehensive description of the Device's design, functionality, and performance characteristics.
1.2 Scope
This specification covers the technical requirements, operational parameters, and safety features of the Glucometer X1. It is intended for use by design engineers, regulatory bodies, quality assurance teams, and other stakeholders involved in the development and assessment of the Device.
2. Device Description
2.1 General Overview
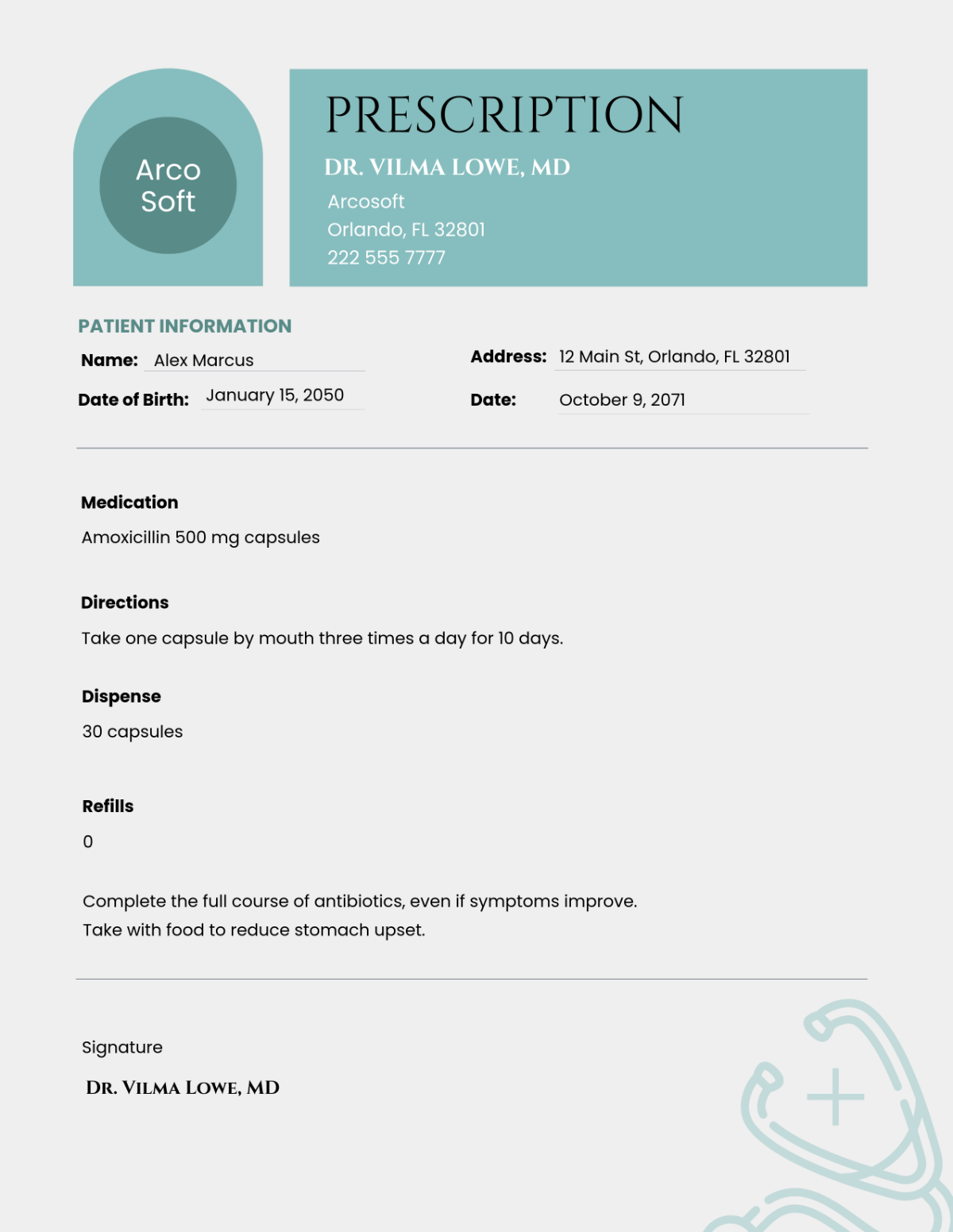
The Glucometer X1 is designed to monitor blood glucose levels in diabetic patients. The Device integrates advanced biosensor technology, ensuring accurate readings and a user-friendly interface for convenient daily use.
2.2 Components
Main Unit: Handheld device with a 2.5-inch LCD screen and integrated touch interface.
Sensors: Glucose sensors with a measurement range of 20 to 600 mg/dL.
Power Supply: Rechargeable lithium-ion battery with a capacity of 2000 mAh.
Accessories: Includes a charging cable, carrying case, and a pack of test strips.
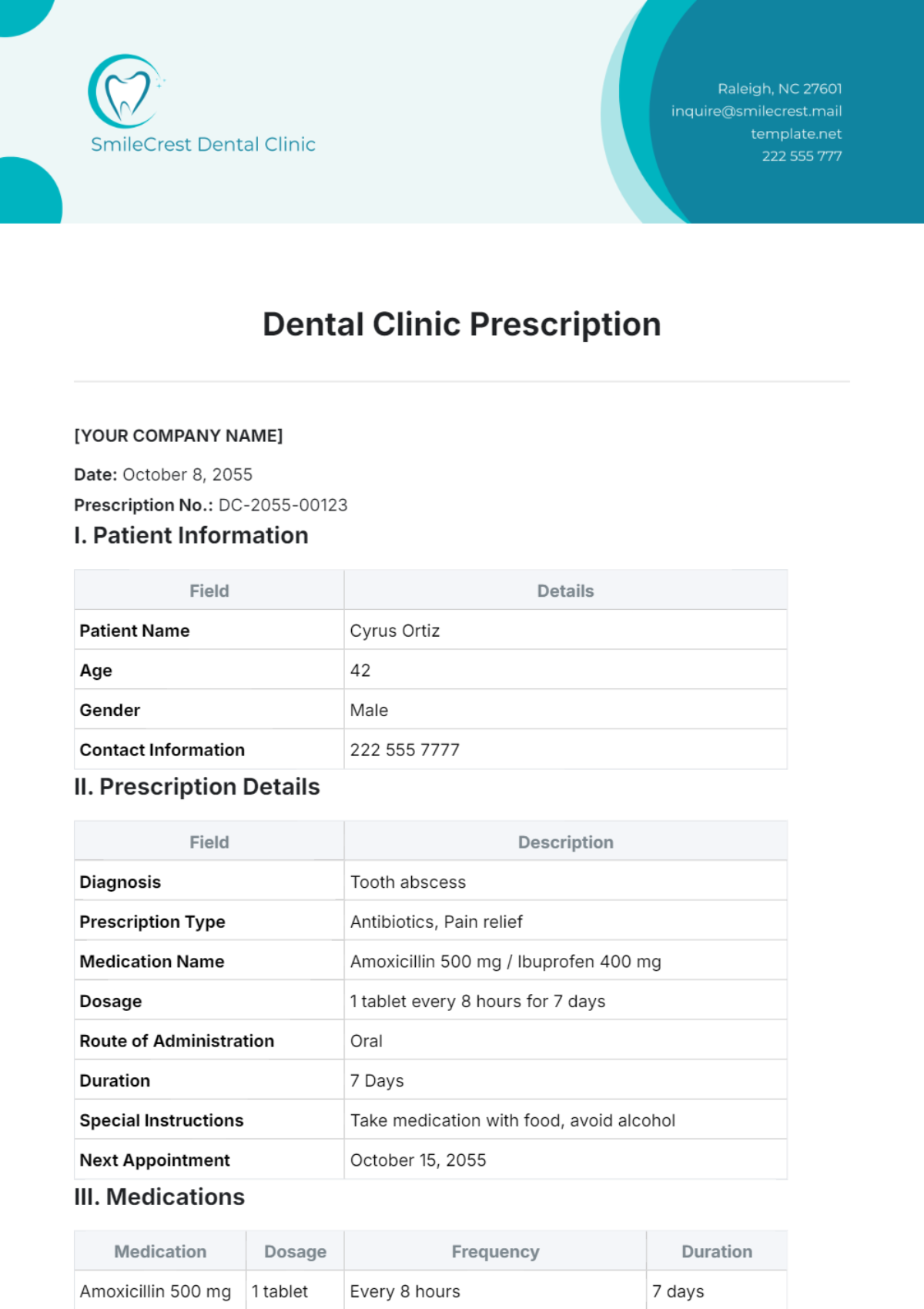
3. Technical Specifications
3.1 Performance Specifications
Accuracy: ±5% of actual value.
Measurement Range: 20 to 600 mg/dL.
Resolution: 1 mg/dL.
Response Time: 5 seconds for measurement.
3.2 Physical Characteristics
Dimensions: 150 x 75 x 30 mm.
Weight: 200 grams.
Material: Medical-grade plastic and stainless steel.
3.3 Environmental Conditions
Operating Temperature: 10°C to 40°C.
Storage Temperature: -20°C to 60°C.
Humidity: 10% to 90% relative humidity, non-condensing.
3.4 Power Requirements
Power Source: Rechargeable lithium-ion battery.
Battery Life: 48 hours of continuous operation.
Charging Time: 2 hours.
4. Functional Requirements
4.1 User Interface
Display: 2.5-inch LCD screen with backlight for easy reading in various lighting conditions.
Controls: Touchscreen with on-screen buttons for intuitive navigation.
Indicators: LED indicators for power, status, and low battery alerts.
4.2 Data Management
Data Storage: Internal memory capable of storing up to 1000 readings.
Data Transfer: Bluetooth 5.0 and USB connectivity for seamless data transfer to compatible devices.
Software: Compatible with iOS and Android operating systems for data synchronization and analysis.
5. Safety and Compliance
5.1 Safety Features
Overload Protection: Automatic shutdown in case of overcurrent to prevent damage.
Shock Resistance: Designed to withstand a 1-meter drop.
User Warnings: Audible alarms and visual indicators for error conditions and operational issues.
5.2 Regulatory Compliance
Standards: Conforms to ISO 13485 and IEC 60601 standards.
Certifications: CE Mark and FDA Approval.
Risk Management: Comprehensive hazard analysis and risk control measures are in place to ensure user safety.
6. Manufacturing and Quality Control
6.1 Manufacturing Process
Production Facilities: Manufactured in an ISO 13485-certified facility to ensure high-quality standards.
Quality Assurance: In-process inspection and final testing to maintain device performance and reliability.
6.2 Documentation
User Manual: Comprehensive guide included with detailed instructions for operation, maintenance, and troubleshooting.
Maintenance Records: Logs for service and repairs are available upon request.
7. Maintenance and Support
7.1 Routine Maintenance
Scheduled Maintenance: Recommended every 6 months to ensure optimal performance.
Cleaning Instructions: Use only approved cleaning agents to avoid damage to the device.
7.2 Technical Support
Contact Information: Support hotline: 1-800-555-1234.
Service Centers: List of authorized service centers available on the company website.
8. Appendices
8.1 Glossary
Biosensor: A device that uses biological molecules to detect specific substances.
Lithium-ion Battery: A type of rechargeable battery known for its high energy density.
8.2 References
ISO 13485: Medical devices — Quality management systems — Requirements for regulatory purposes.
IEC 60601: Medical electrical equipment — Part 1: General requirements for basic safety and essential performance.
For further information or inquiries, please contact:
[YOUR COMPANY NAME]
[YOUR COMPANY EMAIL]
[YOUR COMPANY ADDRESS]
[YOUR COMPANY NUMBER]
[YOUR COMPANY WEBSITE]