Manufacturing Defect Root Cause Analysis
Prepared By: [YOUR NAME]
Date: December 11, 2060
I. Defect Description
The manufacturing defect pertains to the malfunctioning of electric motors used in our automotive line. The defect is characterized by a sudden reduction in motor efficiency, leading to erratic performance and, in some cases, complete failure during operation. This issue has been recurrently observed in models produced between March 2060 and July 2060.
II. Root Cause Investigation
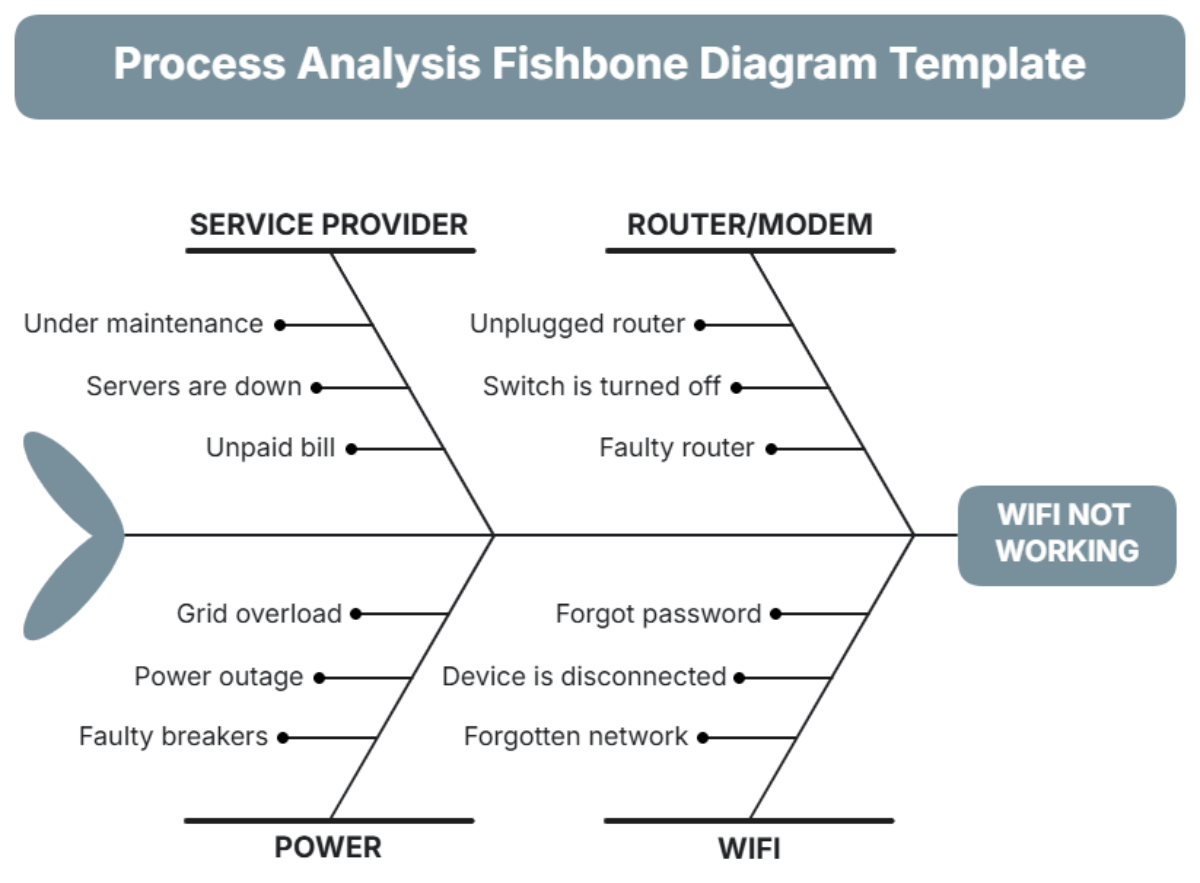
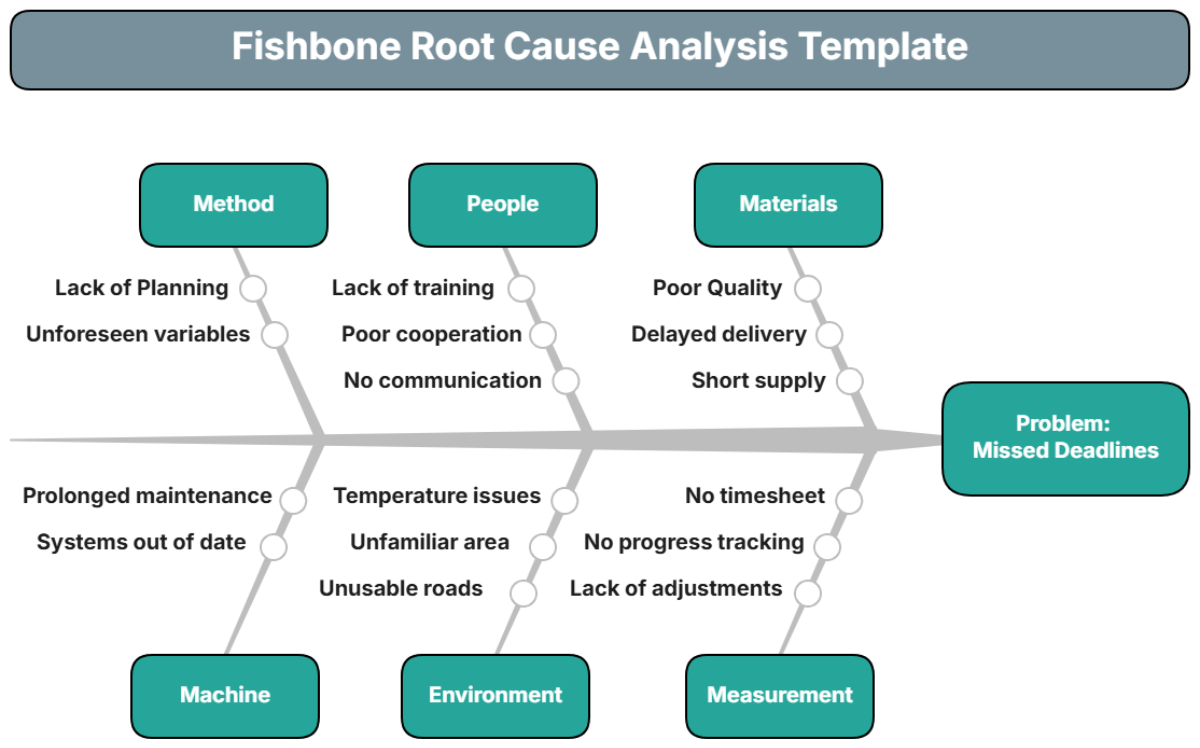
An exhaustive analysis was conducted, focusing on the entire lifecycle of the electric motor, from design to assembly. Key areas of investigation include:
Design Flaws: A review of the motor design revealed potential weaknesses in the rotor's insulation material.
Supplier Quality: Component quality assessments identified inconsistencies in the materials supplied by two vendors.
Manufacturing Process: Discrepancies were found in the thermal treatment process during assembly, likely contributing to material degradation.
Environmental Conditions: Frequency and conditions of storage and transport were examined for irregular handling practices.
It was concluded that the primary root cause was inadequate thermal treatment within the assembly line, leading to the premature failure of insulation in the rotor components.
III. Corrective Actions
To address the root cause identified, the following corrective actions are proposed:
Action | Description |
|---|---|
Process Revision | Revise the thermal treatment process parameters and implement continuous monitoring to ensure compliance with revised specifications. |
Supplier Audits | Conduct in-depth audits of suppliers to ensure that materials meet specified quality standards and consistency. |
Training Enhancement | Develop and roll out an enhanced training program focused on correct handling and processing practices for technical staff. |
IV. Preventive Measures
To prevent the recurrence of similar defects, we propose the following preventative measures:
Implement a robust quality control mechanism at each stage of motor production, including pre-and post-thermal treatment inspections.
Institute regular cross-functional reviews and audits of the production process to ensure ongoing adherence to quality standards.
Enhance communication channels with suppliers to facilitate proactive detection and resolution of material quality issues.
Develop a predictive maintenance schedule for machinery used in thermal processing to minimize potential operational anomalies.
V. Impact Analysis
The impact of the defect on production, cost, and quality has been significant:
Production: The defect caused a temporary halt in production and the need for rework, delaying the delivery schedules by up to 20%.
Cost: Additional costs incurred include overtime labor for inspections, reprocessing of materials, and increased warranty claims.
Quality: The perceived dip in quality has negatively impacted customer satisfaction, with a 15% increase in returns and complaints.
VI. Timeline/Responsibility
A structured timeline is essential for the seamless implementation of corrective actions, as detailed below:
Action | Deadline | Responsible Party |
|---|---|---|
Complete Process Revision | December 2060 | Head of Manufacturing |
Conduct Supplier Audits | January 2061 | Supply Chain Manager |
Roll Out Training Program | March 2061 | Human Resources Director |
Implement Quality Control Mechanism | April 2061 | Quality Assurance Manager |