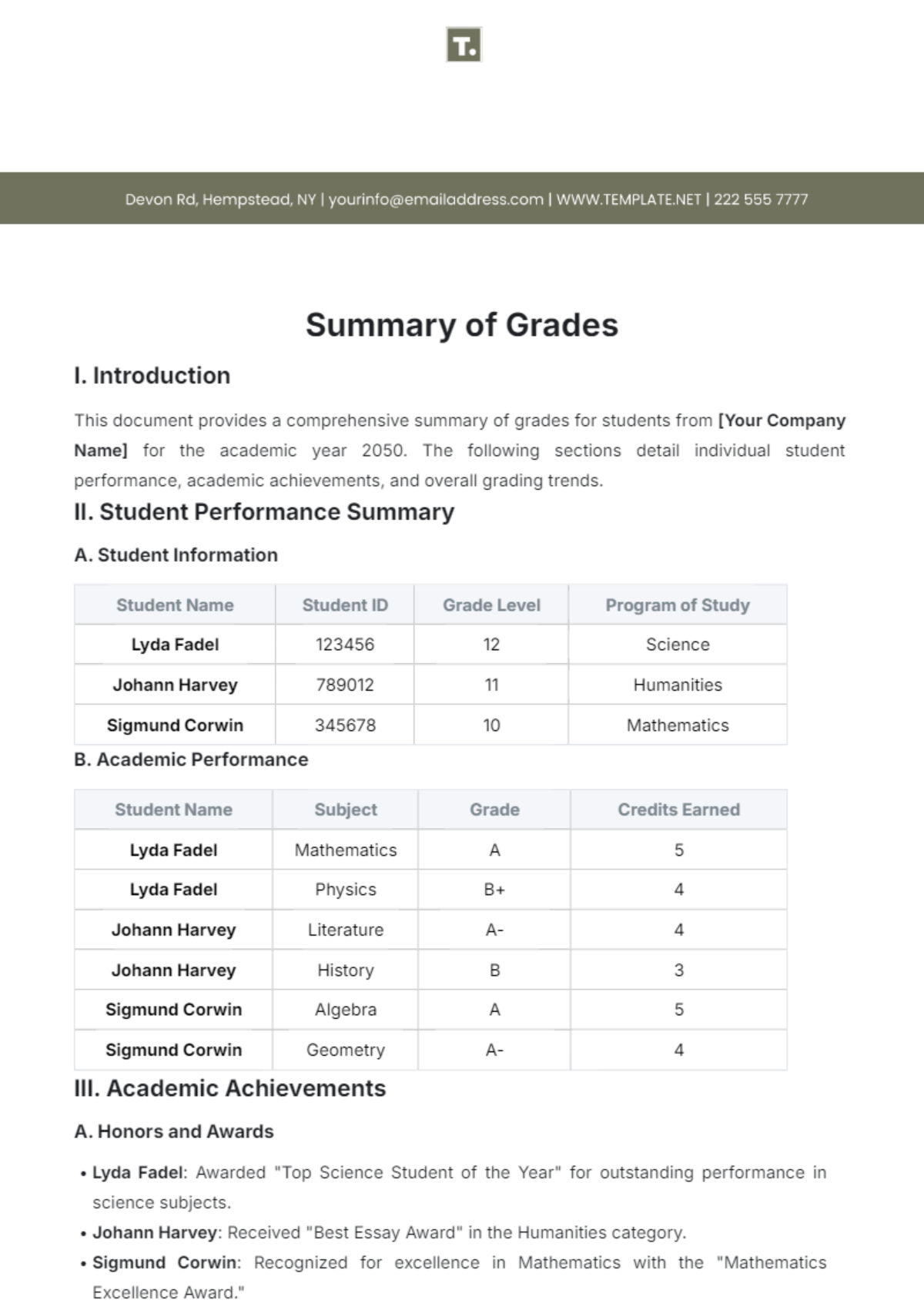
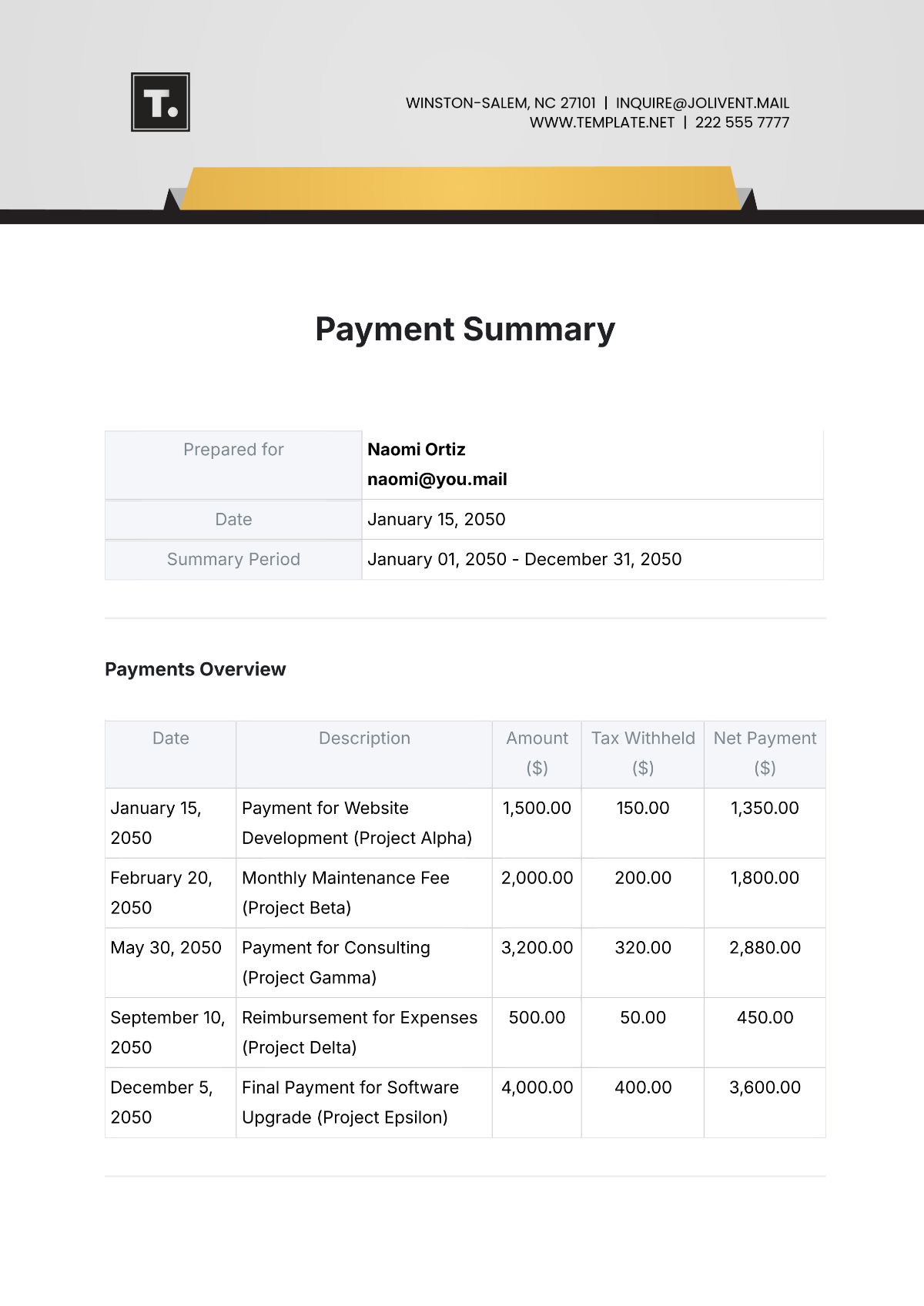
Process Validation Summary
Date: [DATE]
Project: Validation of Manufacturing Process for [PHARMACEUTICAL PRODUCT]
Specialist: [YOUR NAME]
Regulatory Agencies: [LIST OF RELEVANT REGULATORY AGENCIES]
Introduction:
This Process Validation Summary outlines the comprehensive validation conducted on the manufacturing process for [PHARMACEUTICAL PRODUCT]. The validation process adheres strictly to regulatory standards and guidelines, ensuring the safety, efficacy, and quality of the pharmaceutical product.
Key Findings:
Process Parameters:
All critical process parameters were identified and closely monitored throughout the validation process.
Parameters such as [TEMPERATURE, PRESSURE, AGITATION SPEED, REACTION TIME] remained within predetermined limits consistently.
Equipment Qualification:
Rigorous qualification and validation of manufacturing equipment were carried out in accordance with regulatory requirements.
Calibration schedules were strictly followed, ensuring the accuracy and reliability of equipment used in the manufacturing process.
Raw Material Testing:
Raw materials utilized in the manufacturing process underwent thorough testing for [IDENTITY, PURITY, POTENCY].
Certificates of Analysis (CoA) from approved suppliers were obtained to verify the quality and compliance of raw materials.
Process Controls:
Robust process control measures were implemented to mitigate risks and ensure product quality.
In-process checks and monitoring procedures were established to detect and address deviations promptly.
Cleaning Validation:
Cleaning validation protocols were validated to ensure effective removal of residues and contaminants from equipment surfaces.
Analytical testing confirmed the adequacy of cleaning procedures and absence of cross-contamination.
Validation Results:
The validation results affirm the consistency and reliability of the manufacturing process for [PHARMACEUTICAL PRODUCT]. Key validation parameters and their corresponding results are summarized below:
Parameter | Acceptance Criteria | Validation Results |
|---|---|---|
[PARAMETER] | [ACCEPTANCE CRITERIA] | [VALIDATION RESULTS] |
Conclusion:
The successful validation of the manufacturing process for [PHARMACEUTICAL PRODUCT] underscores our commitment to ensuring product quality, safety, and efficacy. The adherence to regulatory standards and meticulous validation procedures have validated the reliability and consistency of the manufacturing process. The findings presented in this summary will be submitted to regulatory agencies for review and approval.
Summarized By:
[YOUR NAME]