TransCelerate Protocol
Prepared by: [YOUR NAME]
Email: [YOUR EMAIL]
Company Name: [YOUR COMPANY NAME]
Company Address: [YOUR COMPANY ADDRESS]
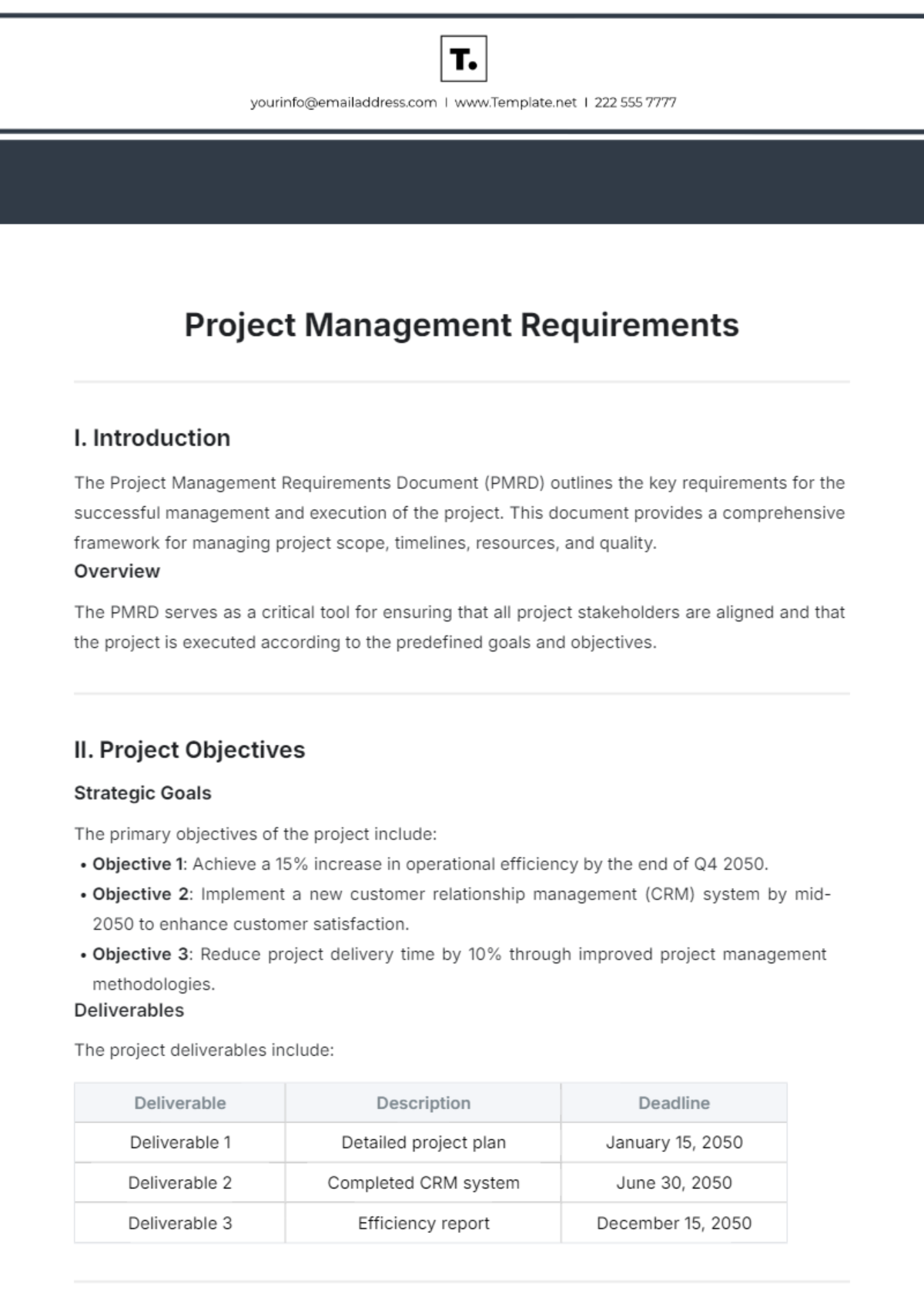
I. Introduction
In the rapidly evolving landscape of clinical research, a well-structured clinical trial design is paramount to ensure the success of investigational therapies. This protocol outlines a comprehensive framework for developing and implementing clinical trials that prioritize patient safety, scientific integrity, and regulatory compliance. By following this standardized approach, researchers can enhance collaboration and streamline processes, ultimately accelerating the journey from research to practice.
II. Objective
The primary objective of this protocol is to establish a standardized clinical trial design that:
Ensures patient safety through rigorous monitoring and adherence to ethical guidelines.
Facilitates data integrity by employing standardized methodologies.
Promotes efficiency in trial operations and resource allocation.
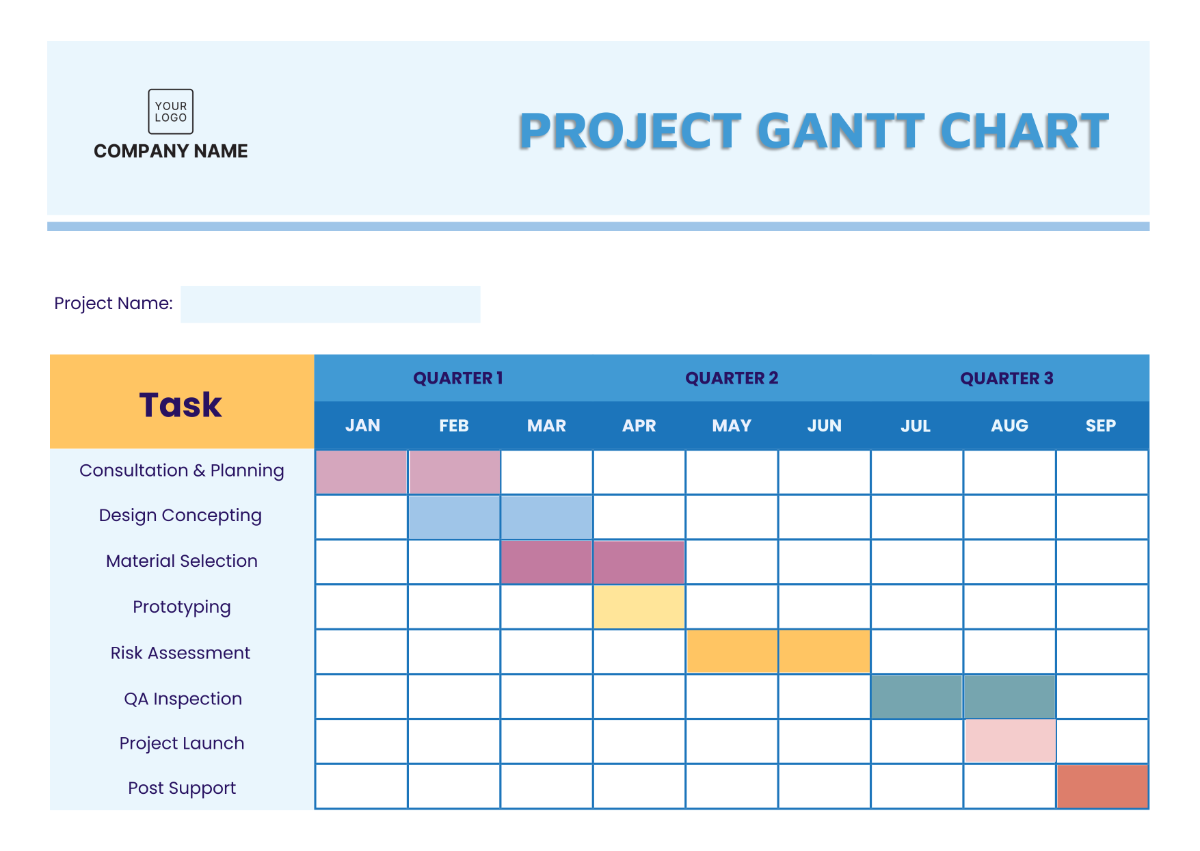
III. Study Design Overview
Study Phase | Design Type | Population | Primary Endpoint | Duration |
|---|---|---|---|---|
Phase I | Open-label | Healthy Volunteers | Safety and Tolerability | January 15, 2050 |
Phase II | Randomized Control | Patients with Condition | Efficacy Measurement | March 20, 2050 |
Phase III | Double-blind | General Population | Overall Survival Rate | June 30, 2050 |
Phase IV | Observational | Post-Market Patients | Long-term Effects | September 10, 2050 |
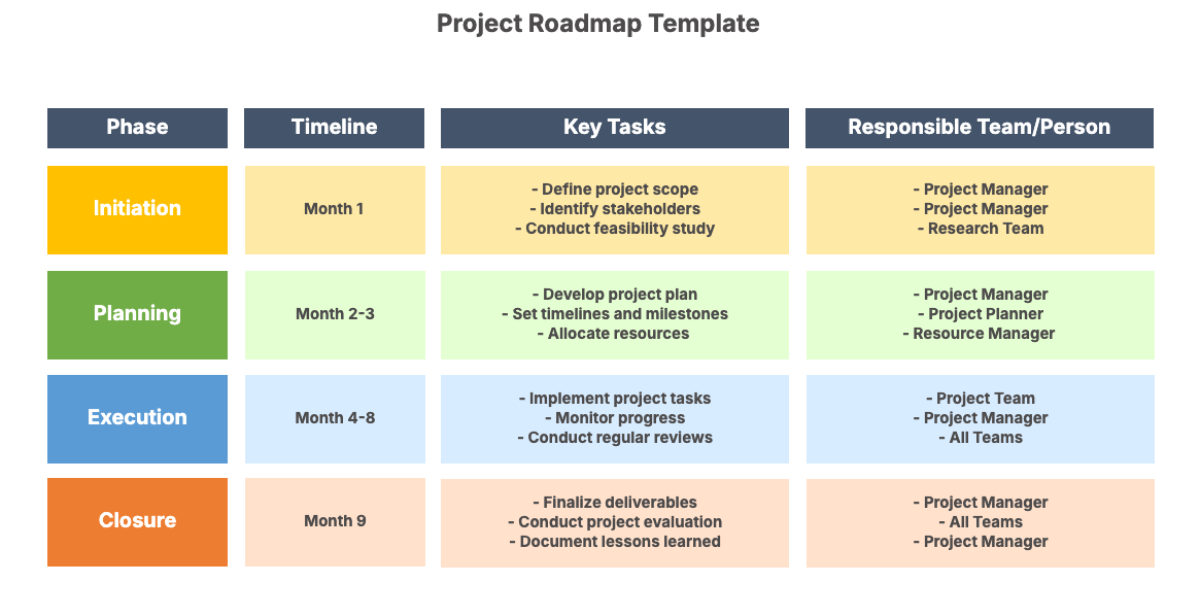
IV. Methodology
This section outlines the methodological framework that will be used to conduct the clinical trial:
Participant Selection: Clearly defined inclusion and exclusion criteria will be established to ensure a representative sample while prioritizing participant safety.
Randomization and Blinding: Where applicable, trials will incorporate randomization and blinding to minimize bias and ensure the reliability of results.
Data Collection and Management: Data will be collected using standardized forms and electronic systems to ensure accuracy and facilitate analysis.
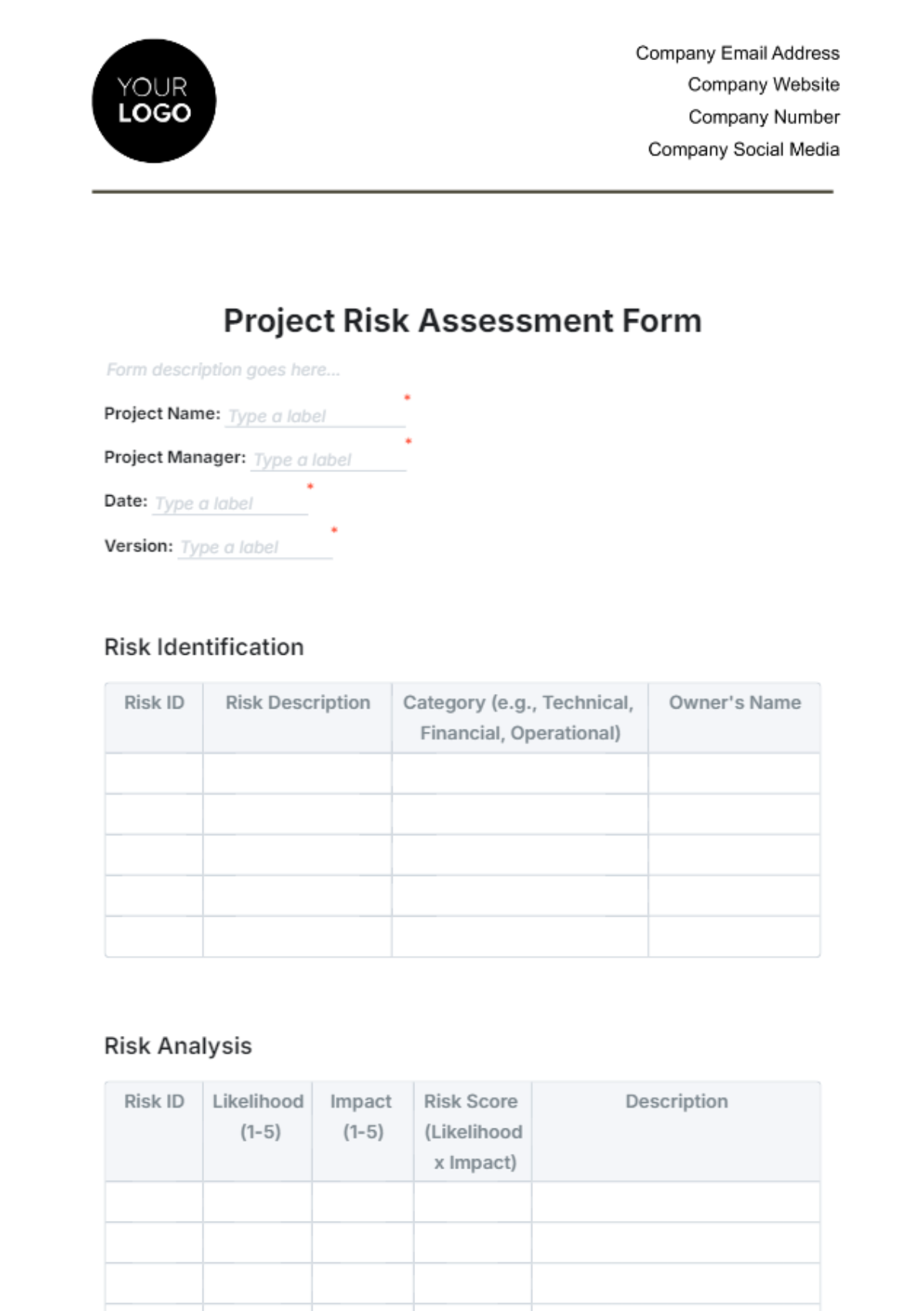
V. Monitoring and Compliance
The trial will incorporate a robust monitoring plan to ensure compliance with regulatory requirements and ethical standards. Regular audits and safety assessments will be conducted throughout the study duration to safeguard participant welfare and data integrity.
VI. Conclusion
By adhering to the guidelines outlined in this TransCelerate Protocol, researchers can achieve a higher degree of consistency and reliability in clinical trial design. This approach not only benefits the research community but also enhances the potential for successful outcomes that ultimately improve patient care and therapeutic advancements.
VII. References
TransCelerate BioPharma Inc. Guidelines for Clinical Trial Design.
FDA (Food and Drug Administration). Clinical Trial Design Guidance.
ICH (International Council for Harmonisation). Good Clinical Practice Guidelines.