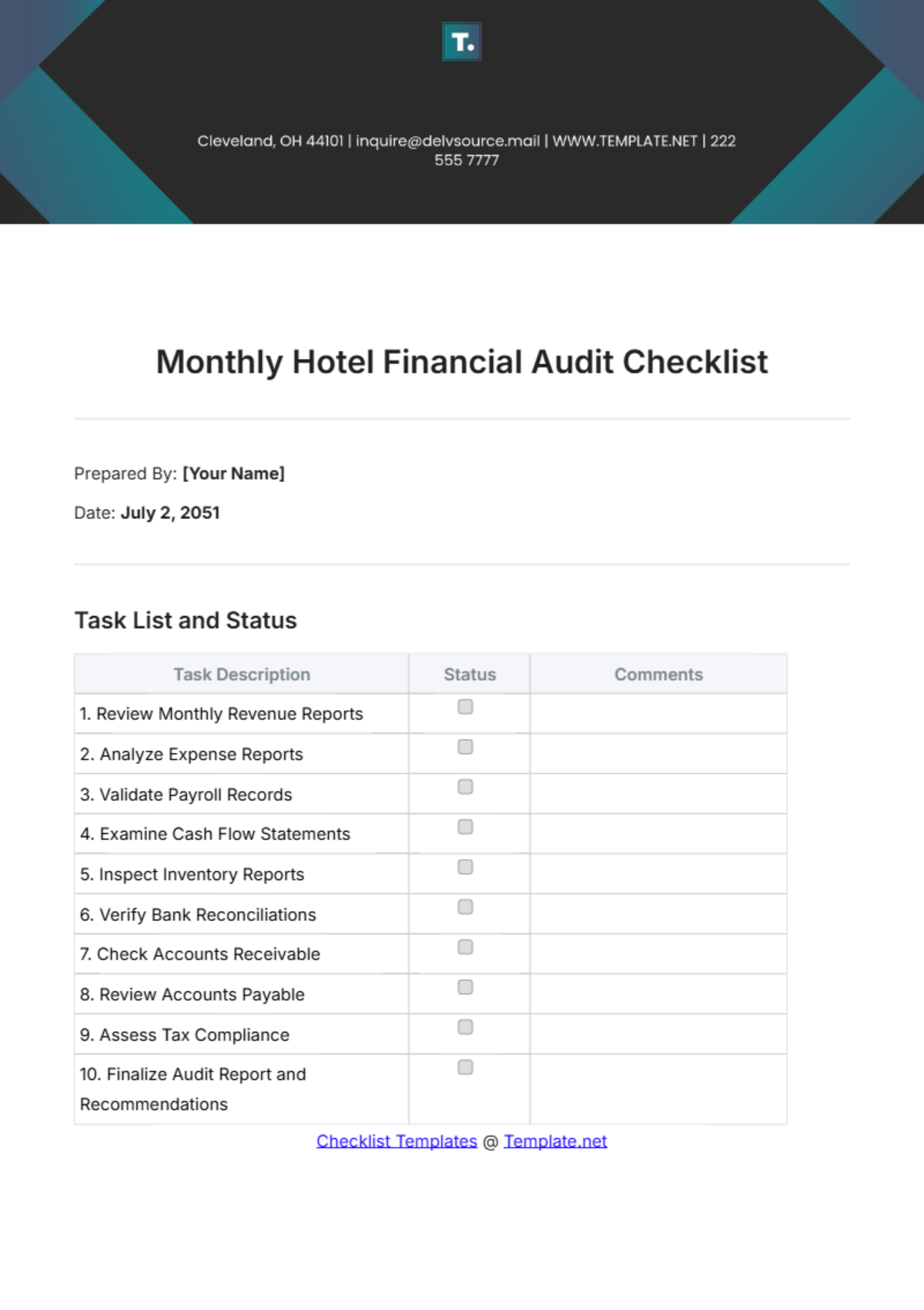
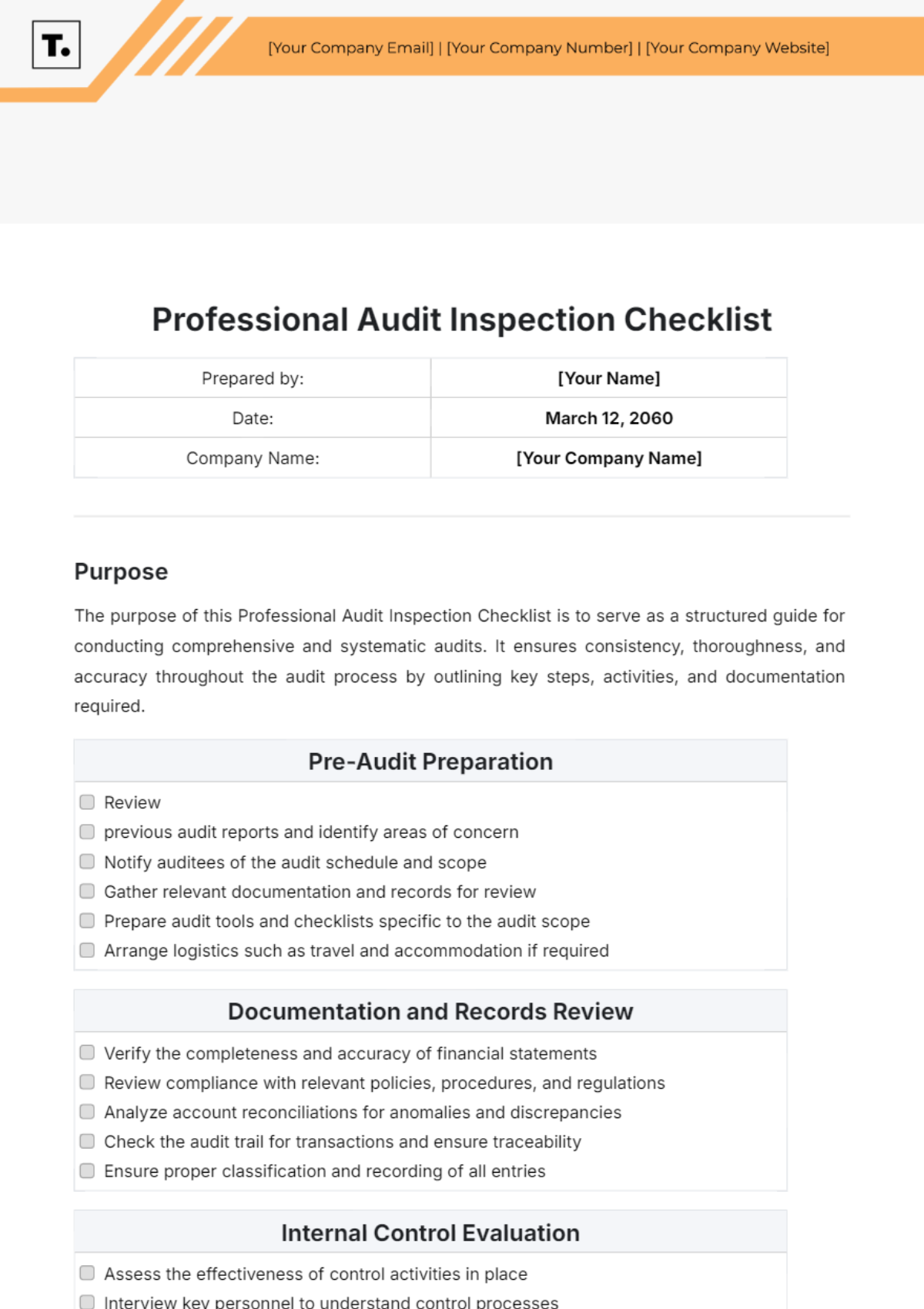
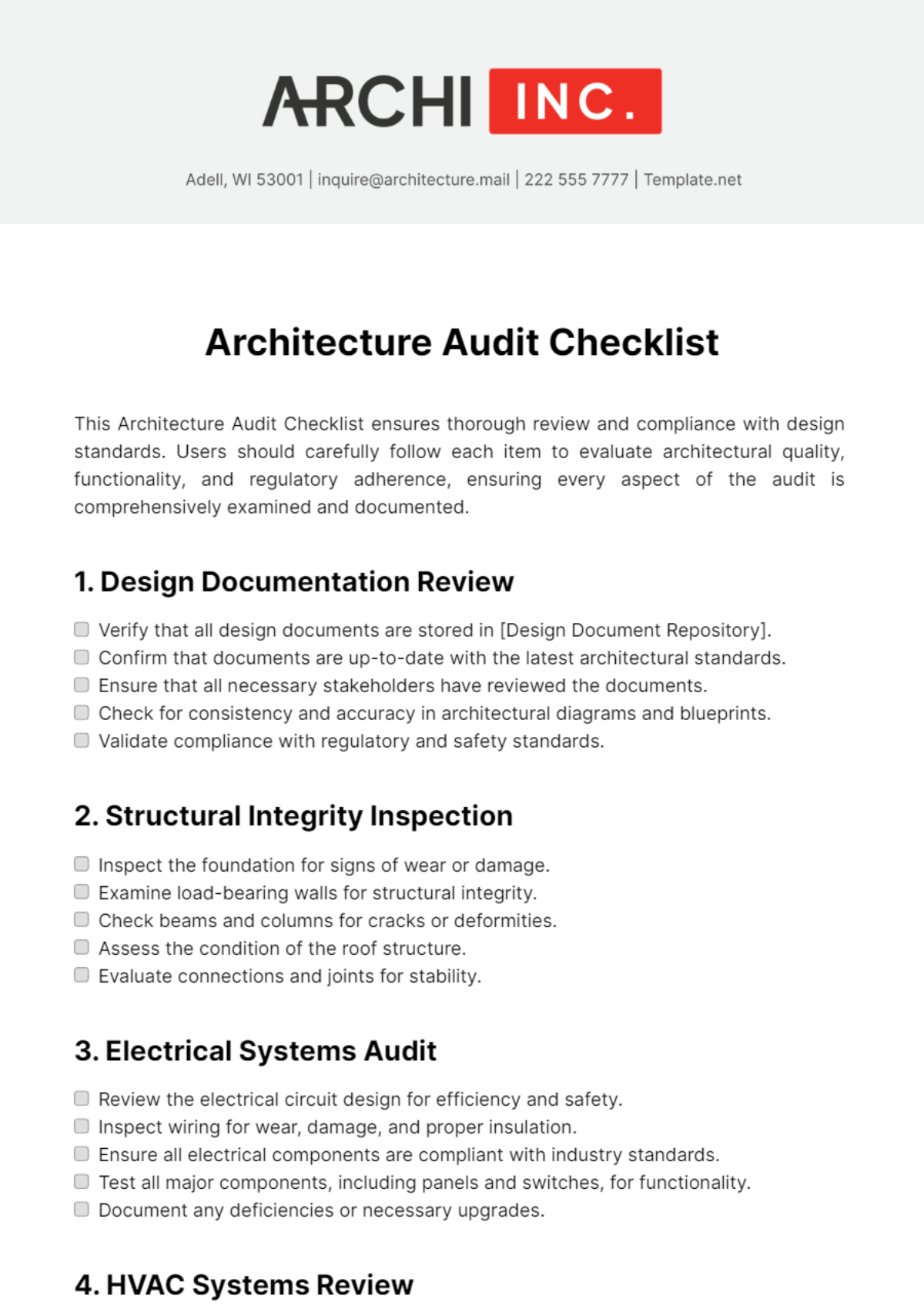
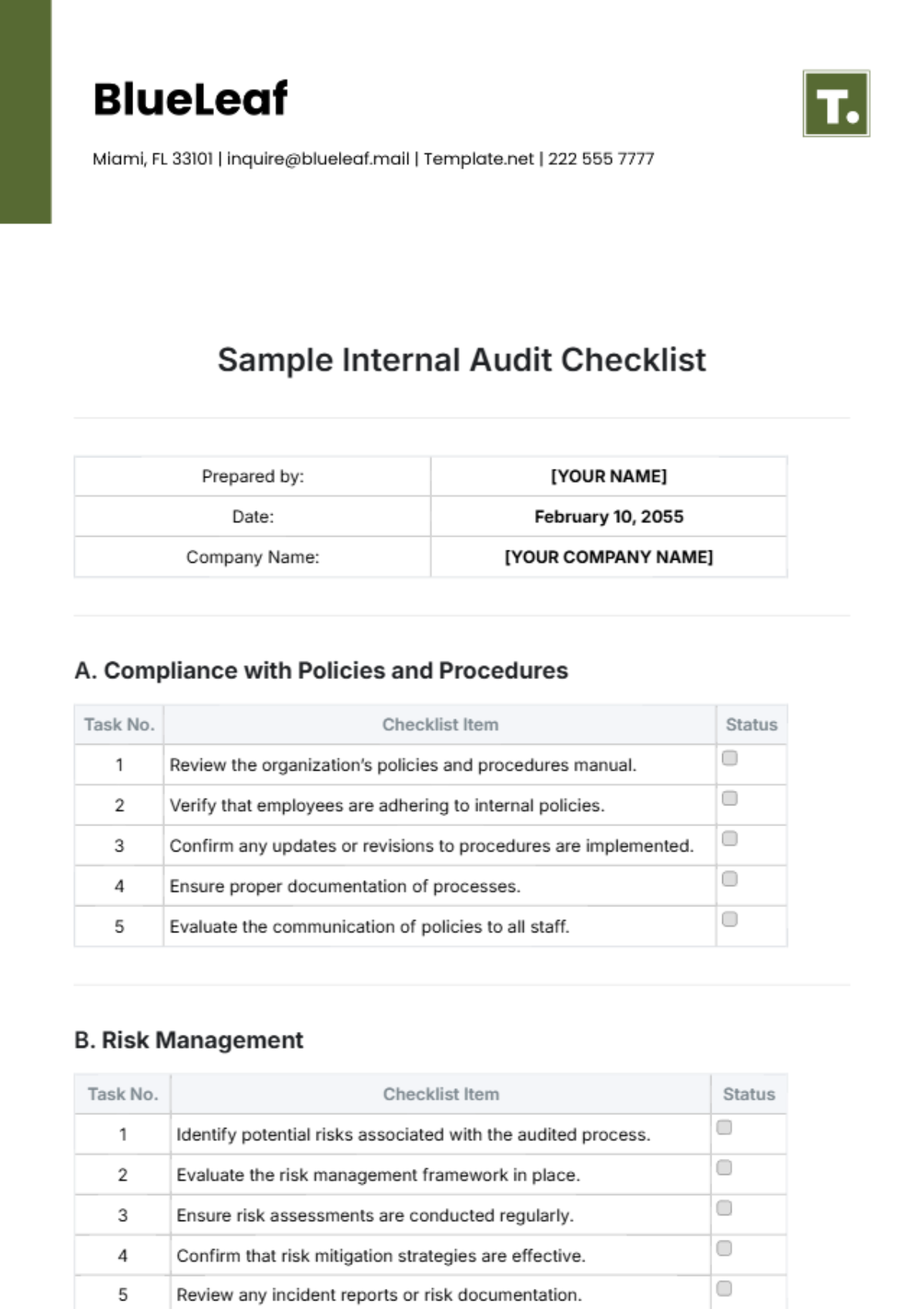
Audit Checklist for Medical Device Facility
Prepared by: | [YOUR NAME] |
Date: | September 4, 2055 |
Company Name: | [YOUR COMPANY NAME] |
I. Regulatory Compliance
Checklist | Status |
|---|---|
Verify facility licensing and registration with applicable regulatory bodies. | |
Ensure all staff qualifications meet regulatory standards. | |
Review adherence to FDA and other relevant medical device regulations. | |
Confirm labeling and promotional materials comply with regulations. |
II. Quality Management System (QMS)
Checklist | Status |
|---|---|
Assess the implementation of the facility's quality management system. | |
Examine document control processes for effectiveness and compliance. | |
Inspect internal audit records and corrective action procedures. | |
Verify regular quality management reviews and updates. |
III. Production and Process Controls
Checklist | Status |
|---|---|
Evaluate cleanliness and maintenance of production equipment. | |
Review validation and control of manufacturing processes. | |
Check the traceability of raw materials and components. | |
Ensure process documentation is accurate and up-to-date. |
IV. Risk Management
Checklist | Status |
|---|---|
Audit risk management plans and activities. | |
Ensure risk assessments are conducted and documented. | |
Verify implementation and monitoring of risk control measures. | |
Review emergency preparedness and response plans. |
V. Training and Competency
Checklist | Status |
|---|---|
Evaluate training programs and materials. | |
Verify staff competency and certification records. | |
Ensure training is conducted regularly and covers relevant updates. | |
Check the effectiveness of training programs through assessments. |
VI. Facility and Environmental Controls
Checklist | Status |
|---|---|
Inspect facility layout for compliance with safety standards. | |
Review environmental monitoring results and controls. | |
Assess maintenance schedules for facility equipment. | |
Verify that waste management procedures are in place and followed. |
VII. Customer Complaints and Product Quality
Checklist | Status |
|---|---|
Review customer feedback and complaint-handling procedures. | |
Audit records of product quality issues and resolutions. | |
Ensure there is a systematic approach to product recalls. | |
Verify improvements are made based on customer feedback. |