Free Prescription Drug Ad Compliance Advertising Checklist

This checklist serves as a guide for [Your Company Name] to ensure all prescription drug advertisements comply with regulatory standards. Follow each step carefully to maintain legal and ethical integrity in your advertising practices.
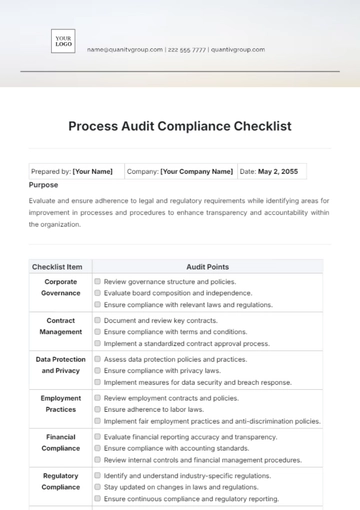
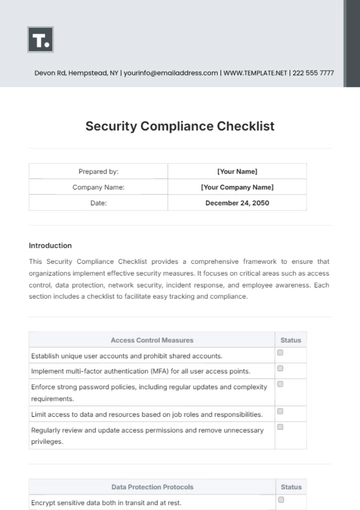
1. Legal and Regulatory Compliance
Confirm adherence to all FDA guidelines regarding content, fair balance, and presentation in the advertisement.
Ensure compliance with both state and federal laws, including any specific regional advertising restrictions.
Check that the ad does not promote off-label use of the drug, which is prohibited under FDA regulations.
If the ad includes comparative claims with other drugs, verify they are supported by scientific evidence and presented fairly.
Consider consulting with regulatory experts or legal counsel to validate compliance, especially for complex or high-profile campaigns.
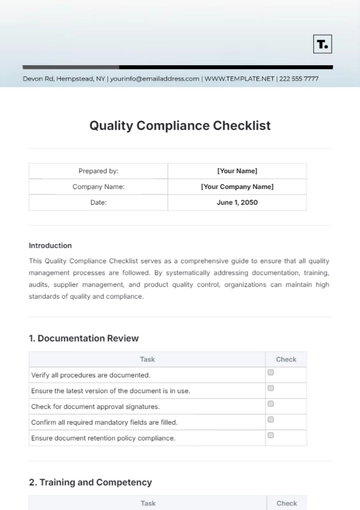
2. Accuracy and Non-Misleading Content
All statements must be supported by clinical studies or approved drug labeling.
Information should be easily understandable by the general public, avoiding medical jargon where possible.
Ensure that any quantifiable claims are clearly supported by data, with any limitations or study contexts adequately disclosed.
Maintain records of the data or studies that substantiate the claims made in the ad.
Present risk information in a manner that is easily noticeable and understandable by consumers.
Avoid overstating the effectiveness or safety of the drug.
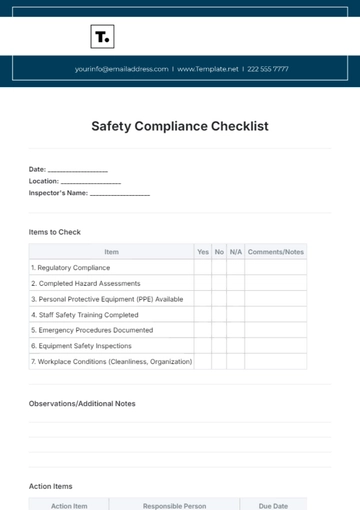
3. Balanced Presentation of Risks and Benefits
Both positive and negative aspects of the drug should be presented with equal prominence.
Ensure that the most serious and frequent risks are prominently displayed.
Present risks within the context of the drug's benefits, helping consumers make informed decisions.
Avoid presentation formats or techniques that minimize the visibility or impact of risk information.
Ensure that risk information is consistent with the drug’s FDA-approved labeling.
If patient testimonials are used, ensure they do not misrepresent or downplay the risks.
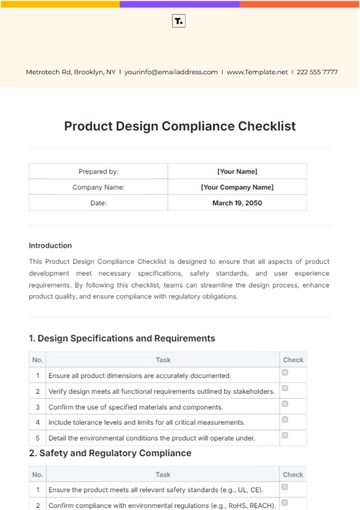
4. Appropriateness of Language and Imagery
Language should be appropriate for a medical product and should not include colloquialisms or slang.
Visuals should accurately represent the drug’s intended use and should not be overly dramatic or emotionally manipulative.
Be culturally sensitive and avoid stereotypes or generalizations in language and imagery.
Ensure that the language used is understandable to the average consumer without medical training.
If endorsements are used, ensure they are from credible and qualified medical professionals.
Do not use fear or intimidation as a tactic to promote the drug.
5. Disclosure Requirements
Disclosures must include the drug’s generic name, approved uses, and any significant risks or side effects.
The sponsoring pharmaceutical company should be clearly identified.
Disclosures should be presented in a manner that ensures a fair balance between benefit and risk information.
All disclosures must be clear and conspicuous, easily readable or understandable by the average viewer.
If the ad directs consumers to a website, ensure the website also complies with regulatory standards.
Regularly update disclosures to reflect any new safety information or FDA warnings.
6. Target Audience and Distribution Channels
Ensure the advertisement is suitable for the demographics of the audience, especially in terms of health and age.
Choose channels that are appropriate and do not target an underage or inappropriate audience.
Be mindful of geographic-specific regulations and cultural sensitivities.
Select media channels wisely, considering the nature and sensitivity of the drug being advertised.
Ensure that the ad is suitable for the level of understanding of the intended audience.
Be aware of and comply with any restrictions on certain types of media (e.g., television, radio).
7. Review and Approval Process
Conduct a thorough internal review to ensure compliance before the ad is released.
Maintain detailed records of the review process, approvals, and any modifications made.
Have the ad reviewed by legal and regulatory experts, especially for complex drug products.
Establish a clear process for revising and re-evaluating advertisements based on feedback or updated regulations.
Keep documentation organized and readily available for regulatory audits or inquiries.
Regularly train marketing and compliance teams on the latest advertising regulations and best practices.
Prepared by:
[Your Name]
[Your Job Title]
[Your Company Name]
[Your Email]

Date: [Date]
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Unlock precision and compliance with Template.net's Prescription Drug Ad Compliance Advertising Checklist Template. Editable and customizable through our Ai Editor Tool, this checklist ensures adherence to regulatory standards in pharmaceutical advertising. Safeguard your campaigns with ease, navigating complexities effortlessly with Template.net's innovative solution.
You may also like
- Cleaning Checklist
- Daily Checklist
- Travel Checklist
- Self Care Checklist
- Risk Assessment Checklist
- Onboarding Checklist
- Quality Checklist
- Compliance Checklist
- Audit Checklist
- Registry Checklist
- HR Checklist
- Restaurant Checklist
- Checklist Layout
- Creative Checklist
- Sales Checklist
- Construction Checklist
- Task Checklist
- Professional Checklist
- Hotel Checklist
- Employee Checklist
- Moving Checklist
- Marketing Checklist
- Accounting Checklist
- Camping Checklist
- Packing Checklist
- Real Estate Checklist
- Cleaning Checklist Service
- New Employee Checklist
- Food Checklist
- Home Inspection Checklist
- Advertising Checklist
- Event Checklist
- SEO Checklist
- Assessment Checklist
- Inspection Checklist
- Baby Registry Checklist
- Induction Checklist
- Employee Training Checklist
- Medical Checklist
- Safety Checklist
- Site Checklist
- Job Checklist
- Service Checklist
- Nanny Checklist
- Building Checklist
- Work Checklist
- Office Checklist
- Training Checklist
- Website Checklist
- IT and Software Checklist
- Performance Checklist
- Project Checklist
- Startup Checklist
- Education Checklist
- Home Checklist
- School Checklist
- Maintenance Checklist
- Planning Checklist
- Manager Checklist
- Wedding Checklist
- Vehicle Checklist
- Travel Agency Checklist
- Vehicle Inspection Checklist
- Interior Design Checklist
- Backpacking Checklist
- Business Checklist
- Legal Checklist
- Nursing Home Checklist
- Weekly Checklist
- Recruitment Checklist
- Salon Checklist
- Baby Checklist
- Equipment Checklist
- Trade Show Checklist
- Party Checklist
- Hospital Bag Checklist
- Evaluation Checklist
- Agency Checklist
- First Apartment Checklist
- Hiring Checklist
- Opening Checklist
- Small Business Checklist
- Rental Checklist
- College Dorm Checklist
- New Puppy Checklist
- University Checklist
- Building Maintenance Checklist
- Work From Home Checklist
- Student Checklist
- Application Checklist