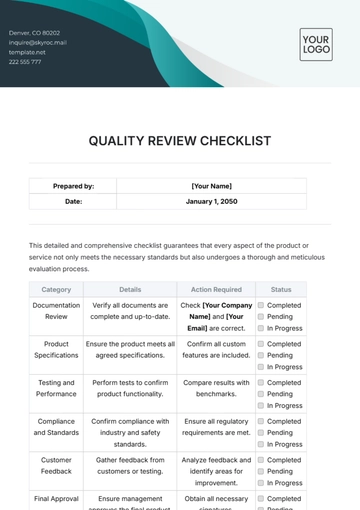
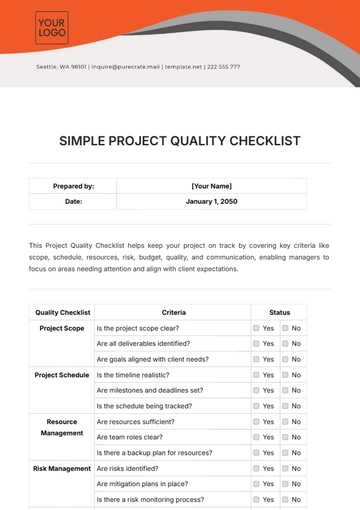
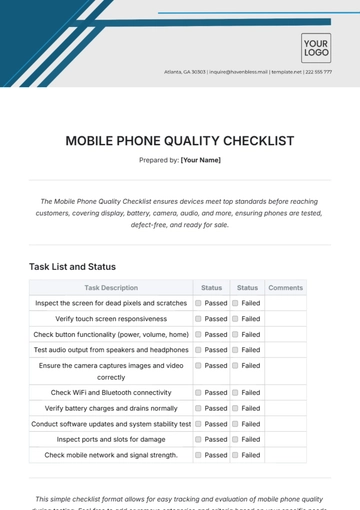
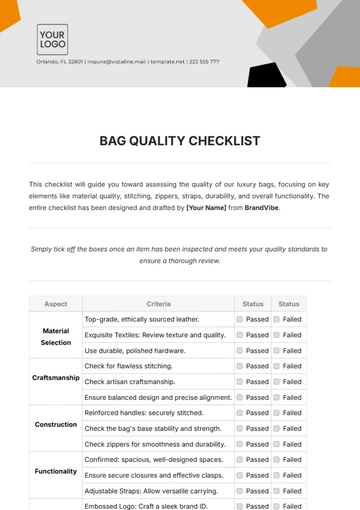
Free Pharmaceutical Quality Checklist

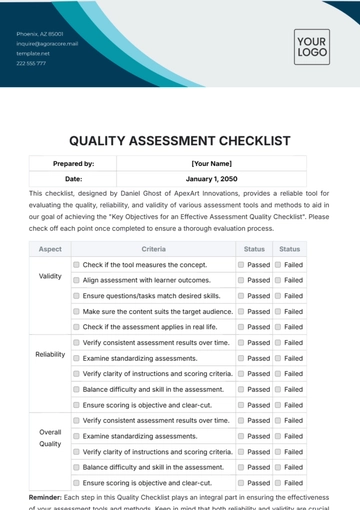
Prepared by: | [Your Name] |
Date: | January 1, 2050 |
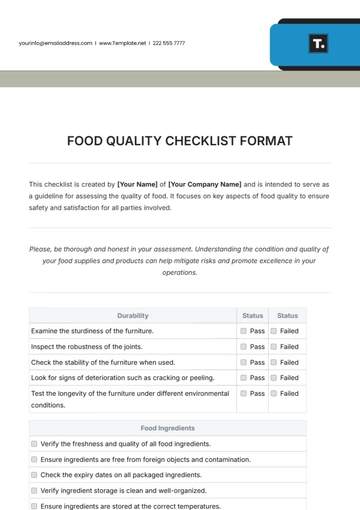
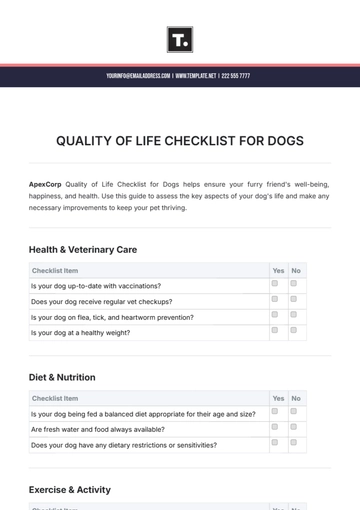
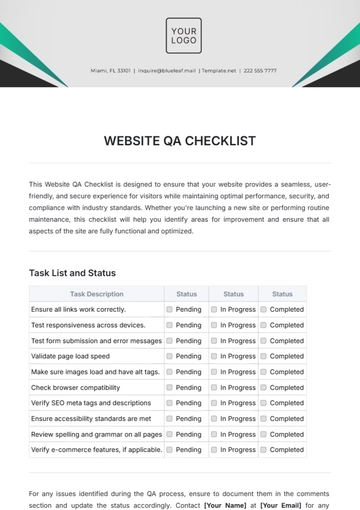
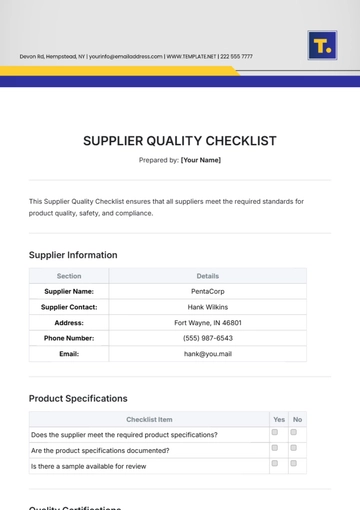
Ensuring top-notch quality in pharmaceutical manufacturing is vital for safety, efficacy, and compliance. This checklist guides you in evaluating and enhancing your QA processes, from raw materials to product distribution, ensuring adherence to regulatory and industry standards. It’s customizable to fit your company's specific needs.
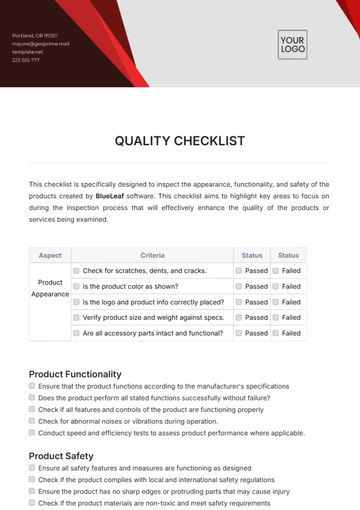
Quality Assurance Procedures
Batch Documentation: Ensure that proper documentation is in place for each batch of product, including production and testing records.
Manufacturing Process Review: Review manufacturing processes to ensure they align with industry standards and internal SOPs (Standard Operating Procedures).
Equipment Maintenance: Conduct regular calibration and maintenance checks for all critical equipment, such as mixers, filling machines, and packaging lines.
Raw Materials and Components
Raw Material Verification: Verify the authenticity and quality of raw materials before use in the manufacturing process.
Supplier Qualification: Confirm that suppliers meet the required quality certifications (e.g., ISO 9001, GMP-certified).
Material Handling Procedures: Ensure that all raw materials are handled according to hygiene and safety standards.
Manufacturing Process
Facility Cleanliness: Inspect equipment and facilities for cleanliness and adherence to safety standards.
Environmental Monitoring: Monitor environmental conditions (temperature, humidity, etc.) during manufacturing.
Batch Record Maintenance: Ensure that batch records are accurately maintained and updated with every change or correction.
Testing and Inspection
In-Process Testing: Perform routine in-process testing for product characteristics such as potency, sterility, and contamination.
Laboratory Analysis: Review results from all laboratory tests, including chemical assays, microbiological tests, and stability studies.
Deviation Management: Ensure that any deviations from expected quality standards are documented and investigated thoroughly.
Packaging and Labeling
Packaging Standards: Ensure that all packaging meets regulatory standards for safety, hygiene, and tamper-evidence.
Label Accuracy: Verify that labels contain accurate product information such as expiry dates, batch numbers, and dosage instructions.
Distribution and Storage
Storage Conditions: Confirm proper storage conditions for the final product, including temperature and humidity control as per the regulatory guidelines.
Storage Area Segregation: Ensure that products are stored in segregated areas to avoid cross-contamination or mix-ups.
Shipping Procedures: Review shipping and distribution procedures to ensure product integrity is maintained during transit.
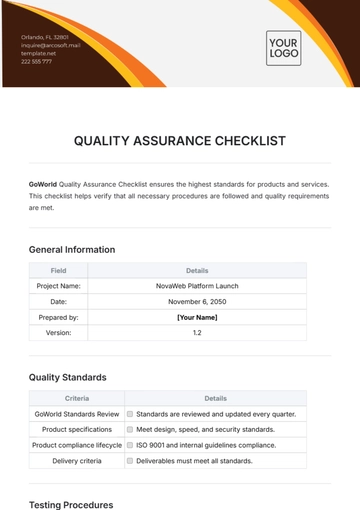
Compliance and Documentation
Regulatory Approvals: Ensure that all necessary regulatory approvals and certifications are in place, including from the FDA, EMA, or other local authorities.
Record Keeping: Maintain up-to-date records of inspections, audits, and corrective actions, ensuring compliance with all legal requirements.
Internal and External Audits: Conduct regular internal and external audits to assess adherence to quality standards and identify areas for improvement.
Use this Pharmaceutical Quality Checklist to ensure top-quality standards, maintain product safety, ensure regulatory compliance, and improve your quality management system through consistent updates and reviews.
- 100% Customizable, free editor
- Access 1 Million+ Templates, photo’s & graphics
- Download or share as a template
- Click and replace photos, graphics, text, backgrounds
- Resize, crop, AI write & more
- Access advanced editor
Ensure high standards in your pharmaceutical processes with this Pharmaceutical Quality Checklist Template from Template.net. Fully editable and customizable, this template helps you maintain compliance and quality control with ease. Editable in our Ai Editor Tool, it allows you to tailor each checklist item to fit specific needs, ensuring thorough documentation and effective oversight.
You may also like
- Cleaning Checklist
- Daily Checklist
- Travel Checklist
- Self Care Checklist
- Risk Assessment Checklist
- Onboarding Checklist
- Quality Checklist
- Compliance Checklist
- Audit Checklist
- Registry Checklist
- HR Checklist
- Restaurant Checklist
- Checklist Layout
- Creative Checklist
- Sales Checklist
- Construction Checklist
- Task Checklist
- Professional Checklist
- Hotel Checklist
- Employee Checklist
- Moving Checklist
- Marketing Checklist
- Accounting Checklist
- Camping Checklist
- Packing Checklist
- Real Estate Checklist
- Cleaning Checklist Service
- New Employee Checklist
- Food Checklist
- Home Inspection Checklist
- Advertising Checklist
- Event Checklist
- SEO Checklist
- Assessment Checklist
- Inspection Checklist
- Baby Registry Checklist
- Induction Checklist
- Employee Training Checklist
- Medical Checklist
- Safety Checklist
- Site Checklist
- Job Checklist
- Service Checklist
- Nanny Checklist
- Building Checklist
- Work Checklist
- Office Checklist
- Training Checklist
- Website Checklist
- IT and Software Checklist
- Performance Checklist
- Project Checklist
- Startup Checklist
- Education Checklist
- Home Checklist
- School Checklist
- Maintenance Checklist
- Planning Checklist
- Manager Checklist
- Wedding Checklist
- Vehicle Checklist
- Travel Agency Checklist
- Vehicle Inspection Checklist
- Interior Design Checklist
- Backpacking Checklist
- Business Checklist
- Legal Checklist
- Nursing Home Checklist
- Weekly Checklist
- Recruitment Checklist
- Salon Checklist
- Baby Checklist
- Equipment Checklist
- Trade Show Checklist
- Party Checklist
- Hospital Bag Checklist
- Evaluation Checklist
- Agency Checklist
- First Apartment Checklist
- Hiring Checklist
- Opening Checklist
- Small Business Checklist
- Rental Checklist
- College Dorm Checklist
- New Puppy Checklist
- University Checklist
- Building Maintenance Checklist
- Work From Home Checklist
- Student Checklist
- Application Checklist